Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Complete the mechanism for the keto-enol tautomerization shown using bonds, charges, nonbonding electron pairs, and curved arrows (forward reaction only). Step 1: enol form
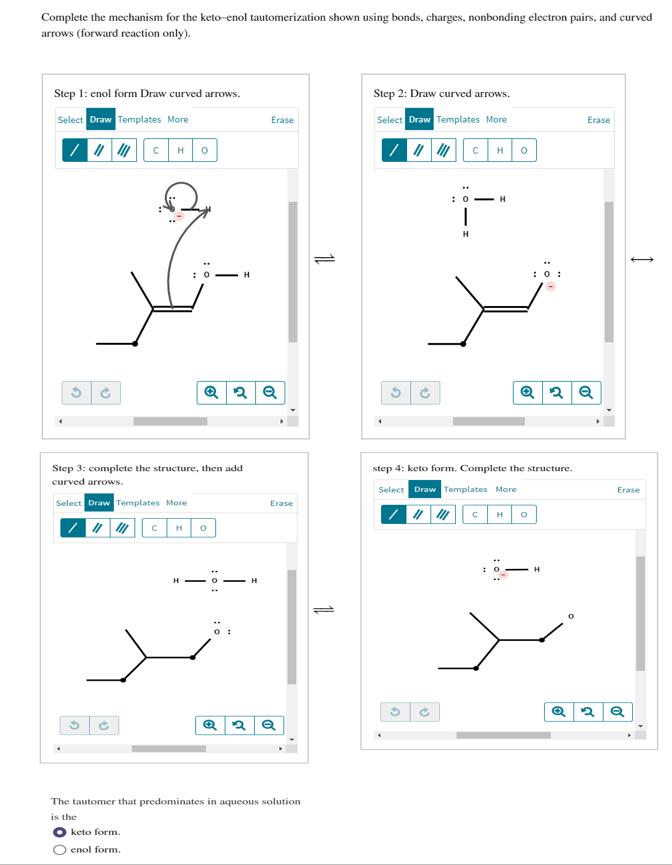
Complete the mechanism for the keto-enol tautomerization shown using bonds, charges, nonbonding electron pairs, and curved arrows (forward reaction only). Step 1: enol form Draw curved arrows. Select Draw Templates More CH : O H Step 2: Draw curved arrows. Erase Select Draw Templates More Q2 Q Step 3: complete the structure, then add curved arrows. Select Draw Templates More H H Q 11 Erase The tautomer that predominates in aqueous solution is the keto form. enol form. P H : 0 H H : 0: Erase Q2 Q step 4: keto form. Complete the structure. Select Draw Templates More C H P H I QQ Erase
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


