Question
Complete the table below for zero, first and simple second order reactions. k, 1/[A]o 1/[A] vs. t -k, In [A]o [A]: vs. t -k,
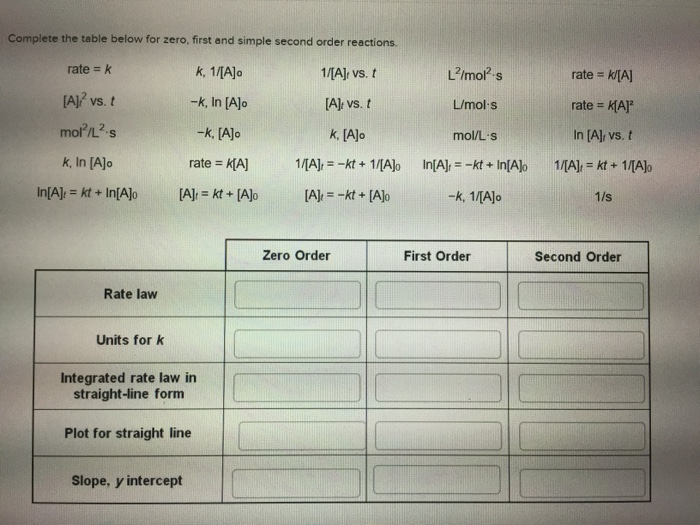
Complete the table below for zero, first and simple second order reactions. k, 1/[A]o 1/[A] vs. t -k, In [A]o [A]: vs. t -k, [A]o k, [A]o rate = K[A] [A] = kt+ [A]o rate = k [A]2 vs. t mol/L-s k, In [A]o In[A] = kt + In[A]o Rate law Units for k Integrated rate law in straight-line form Plot for straight line Slope, y intercept 1/[A] -kt + 1/[A]o [A] = -kt + [A]o Zero Order L/mol-s L/mol s mol/L-s In[A] = -kt + In[A]o -k, 1/[A]o First Order rate= k/[A] rate = K[A] In [A], vs. t 1/[A] = kt + 1/[A]o 1/s Second Order
Step by Step Solution
3.52 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
Rate Law Units for k Entegrate rate low in st l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Horngrens Financial and Managerial Accounting
Authors: Tracie L. Nobles, Brenda L. Mattison, Ella Mae Matsumura
5th edition
9780133851281, 013385129x, 9780134077321, 133866297, 133851281, 9780133851298, 134077326, 978-0133866292
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



