Answered step by step
Verified Expert Solution
Question
1 Approved Answer
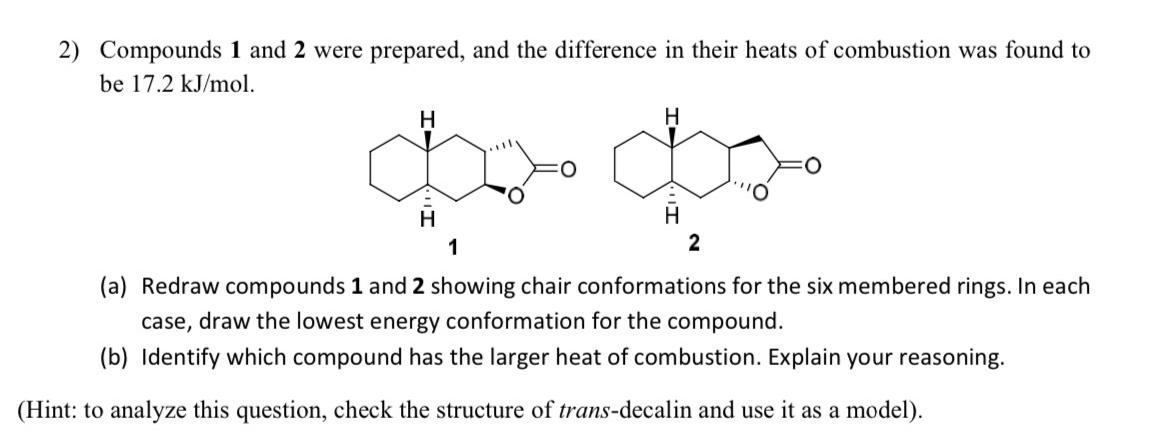
Compounds 1 and 2 were prepared, and the difference in their heats of combustion was found to be 1 7 . 2 k J m
Compounds and were prepared, and the difference in their heats of combustion was found to be
a Redraw compounds and showing chair conformations for the six membered rings. In each case, draw the lowest energy conformation for the compound.
b Identify which compound has the larger heat of combustion. Explain your reasoning.
Hint: to analyze this question, check the structure of transdecalin and use it as a model
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


