Question
Compute the entropy difference between 12 kg of water at 40C and 12 kg of ice at - 10C. (Assume that the heat capacity
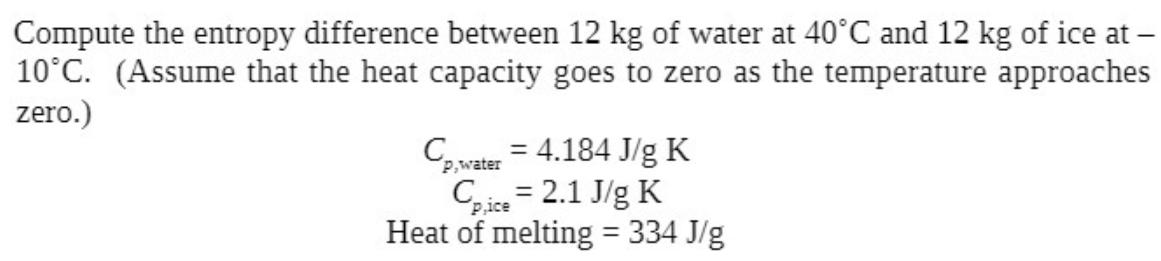
Compute the entropy difference between 12 kg of water at 40C and 12 kg of ice at - 10C. (Assume that the heat capacity goes to zero as the temperature approaches zero.) Cp,water = 4.184 J/g K Cpice = 2.1 J/g K Heat of melting = 334 J/g
Step by Step Solution
There are 3 Steps involved in it
Step: 1
First lets calculate the change in entropy for the water at 40C cooling down to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics for Engineers
Authors: Kenneth A. Kroos, Merle C. Potter
1st edition
1133112862, 978-113311286
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



