Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate consentration of unknown copper(II) sulfate solution. Oxidation half-reaction Metals Reduction Overall half-reaction reaction Standard cell potential (V) Zinc and copper Zn- Zn+ 2e Cu+
Calculate consentration of unknown copper(II) sulfate solution.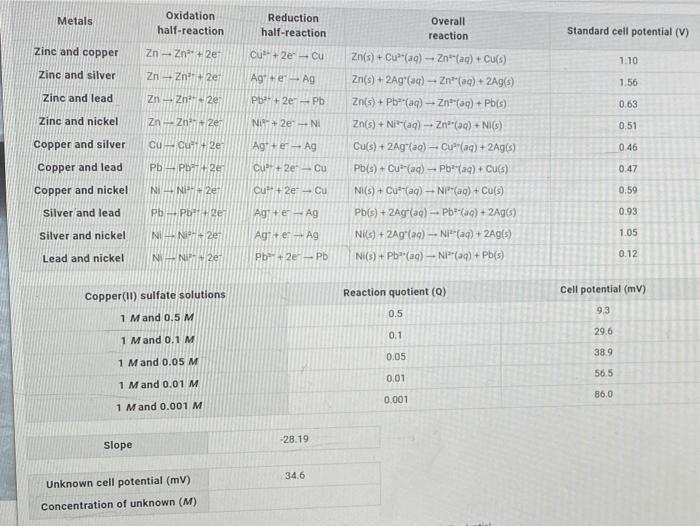
Oxidation half-reaction Metals Reduction Overall half-reaction reaction Standard cell potential (V) Zinc and copper Zn- Zn+ 2e Cu+ 2e Cu Zn(s) + Cu"(aq) - Znt(ag) + Cu(s) 1.10 Zinc and silver Zn - Zn+2e Ag+ e Ag Zn(s) + 2Ag"(aq) - Zn"(aq) + 2Ag(s) 1.56 Zinc and lead Zn - Zn" 2e Pb+ 2e-Pb Zn(s) + Pb"(aq) - Znt(aq) + Pb(s) 0.63 Zinc and nickel Zn- Zn 2e Ni+ 2e NI Zn(s) + Ni"(aq) - Zn"(aq) + NI(s) 0.51 Copper and silver cu Cut+ 2e Agt+e Ag Cu(s) + 2Ag(ag)- Cu"(aq) + 2Ag(s) 0.46 Copper and lead Pb- Pb 2er Cu+ 2e-Cu Pb(s) + Cu (aq) Pb*(aq) + Culs) 0.47 Copper and nickel NI- Ni+ 2e Cu+ 2e Cu NI(s) + Cut"(ag) - NP"(ag) + Cu(s) 0.59 silver and lead Pb-Pb+2e Agt+e-Ag Pb(s) + 2Ag(ag) - Pb(aq) + 2Ag(s) 0.93 Silver and nickel NI-N 2e Ag+er Ag Nis) + 2Ag(aq)- Ni"(aq) + 2Ag(s) 1.05 Lead and nickel Ni- NI 2er Pb+2e Pb Ni(s) + Pb"(aq)- NI"(aq) + Pb(s) 0.12 Reaction quotient (Q) Cell potential (mV) Copper(I) sulfate solutions 9.3 0.5 1 Mand 0.5 M 29.6 0.1 1 Mand 0.1M 38.9 0.05 1 Mand 0.05 M 56.5 0.01 1 Mand 0.01 M 86.0 0.001 1 Mand 0.001 M -28.19 Slope 34.6 Unknown cell potential (mv) Concentration of unknown (M)
Step by Step Solution
★★★★★
3.44 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6368693ed5e33_241692.pdf
180 KBs PDF File
6368693ed5e33_241692.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


