Answered step by step
Verified Expert Solution
Question
1 Approved Answer
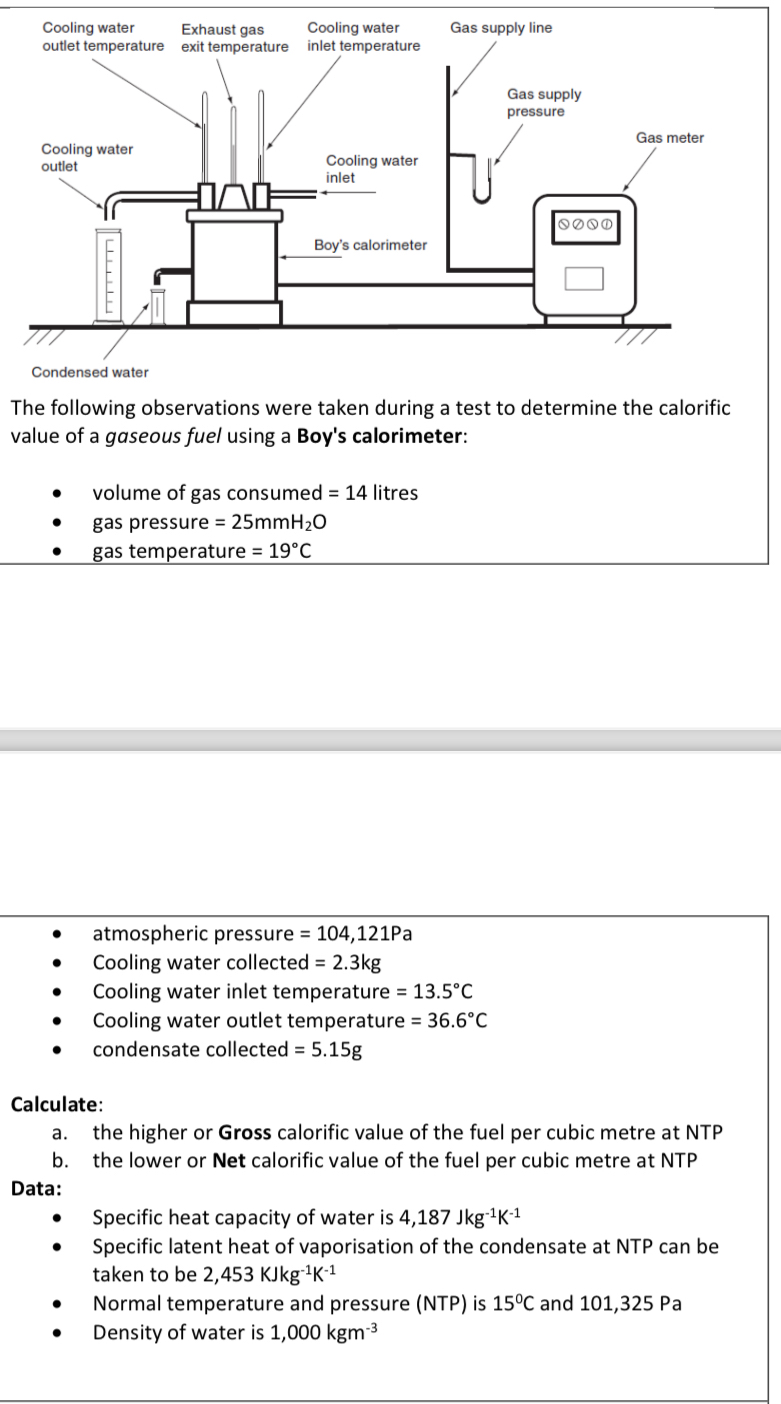
Condensed water The following observations were taken during a test to determine the calorific value of a gaseous fuel using a Boy's calorimeter: , volume
Condensed water
The following observations were taken during a test to determine the calorific value of a gaseous fuel using a Boy's calorimeter:
volume of gas consumed litres
gas pressure
gas temperature
atmospheric pressure
Cooling water collected
Cooling water inlet temperature
Cooling water outlet temperature
condensate collected
Calculate:
a the higher or Gross calorific value of the fuel per cubic metre at NTP
b the lower or Net calorific value of the fuel per cubic metre at NTP
Data:
Specific heat capacity of water is
Specific latent heat of vaporisation of the condensate at NTP can be taken to be
Normal temperature and pressure NTP is and
Density of water is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


