Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Condensing steam Use these numbers for this problem: The specific heat of liquid water is 4186 J/(kg C). The specific heat of water vapor

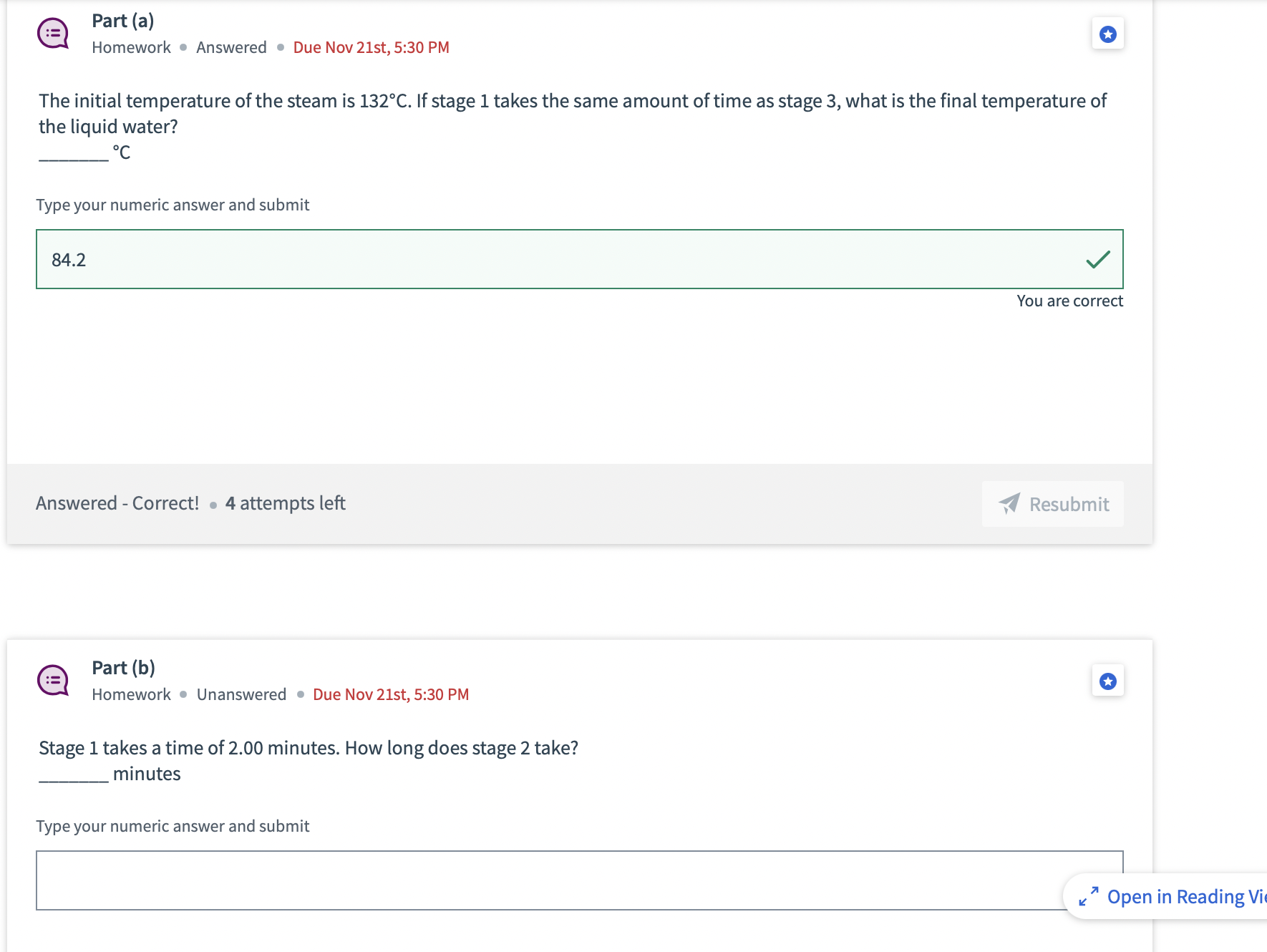
Condensing steam Use these numbers for this problem: The specific heat of liquid water is 4186 J/(kg C). The specific heat of water vapor is 2010 J/(kg C). The latent heat of vaporization of water is 2260000 J/kg. In this problem, a certain mass of steam is run through a condenser, which extracts heat from the water (whether it is a gas or a liquid) at a constant rate. There are three stages to the process: Stage 1 - the water vapor cools as a gas, from its initial temperature to the boiling point. Stage 2 - at the boiling point, the steam condenses to liquid. Stage 3 - the water cools as a liquid to its final temperature. == Part (a) Homework Answered Due Nov 21st, 5:30 PM The initial temperature of the steam is 132C. If stage 1 takes the same amount of time as stage 3, what is the final temperature of the liquid water? C Type your numeric answer and submit 84.2 Answered - Correct! 4 attempts left Part (b) := Homework Unanswered Due Nov 21st, 5:30 PM Stage 1 takes a time of 2.00 minutes. How long does stage 2 take? minutes Type your numeric answer and submit You are correct Resubmit Open in Reading Vi
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


