Answered step by step
Verified Expert Solution
Question
1 Approved Answer
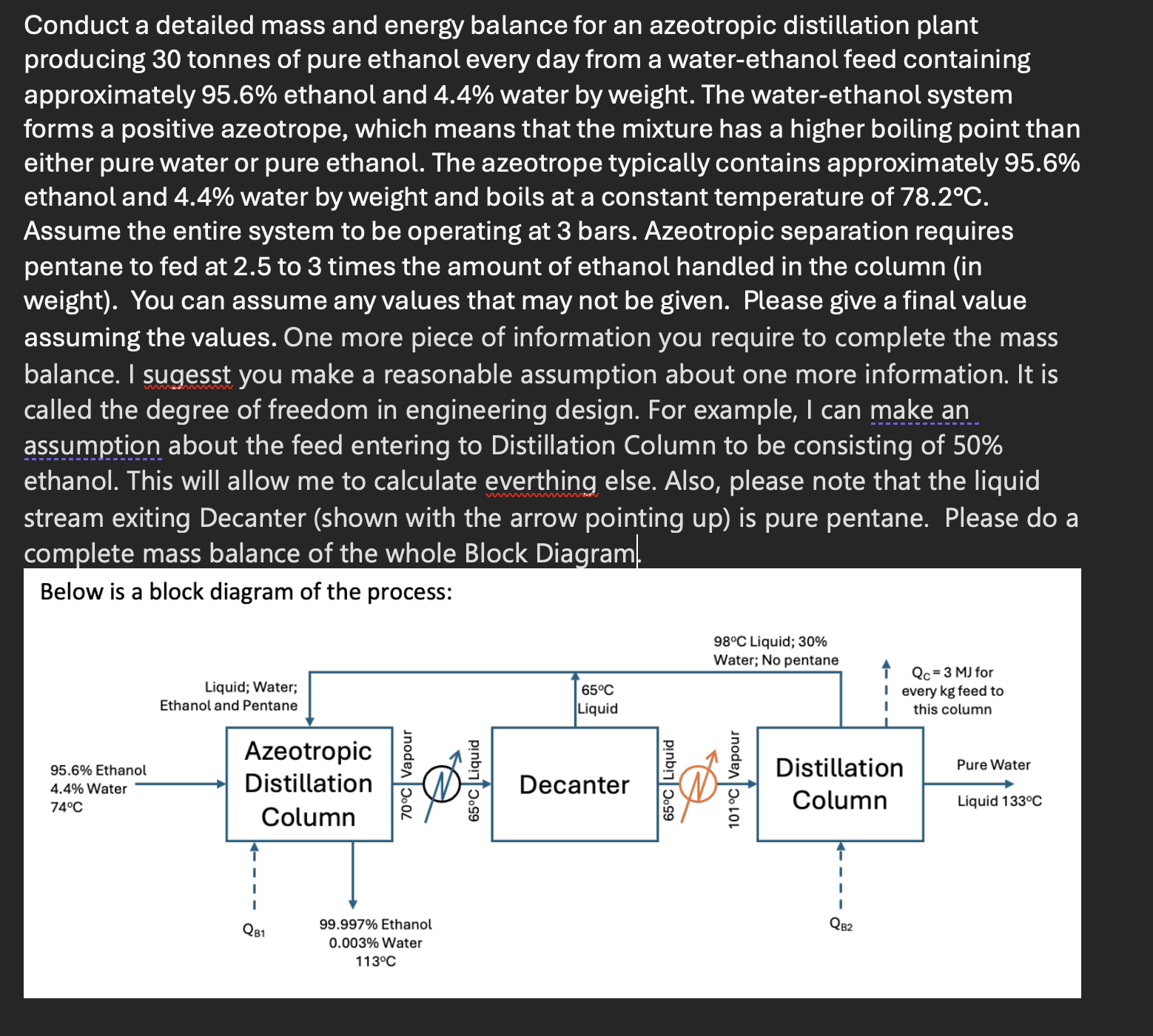
Conduct a detailed mass and energy balance for an azeotropic distillation plant producing 3 0 tonnes of pure ethanol every day from a water -
Conduct a detailed mass and energy balance for an azeotropic distillation plant
producing tonnes of pure ethanol every day from a waterethanol feed containing
approximately ethanol and water by weight. The waterethanol system
forms a positive azeotrope, which means that the mixture has a higher boiling point than
either pure water or pure ethanol. The azeotrope typically contains approximately
ethanol and water by weight and boils at a constant temperature of
Assume the entire system to be operating at bars. Azeotropic separation requires
pentane to fed at to times the amount of ethanol handled in the column in
weight You can assume any values that may not be given. Please give a final value
assuming the values. One more piece of information you require to complete the mass
balance. I sugesst you make a reasonable assumption about one more information. It is
called the degree of freedom in engineering design. For example, I can make an
assumption about the feed entering to Distillation Column to be consisting of
ethanol. This will allow me to calculate everthing else. Also, please note that the liquid
stream exiting Decanter shown with the arrow pointing up is pure pentane. Please do a
complete mass balance of the whole Block Diagram.
Below is a block diagram of the process:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


