Answered step by step
Verified Expert Solution
Question
1 Approved Answer
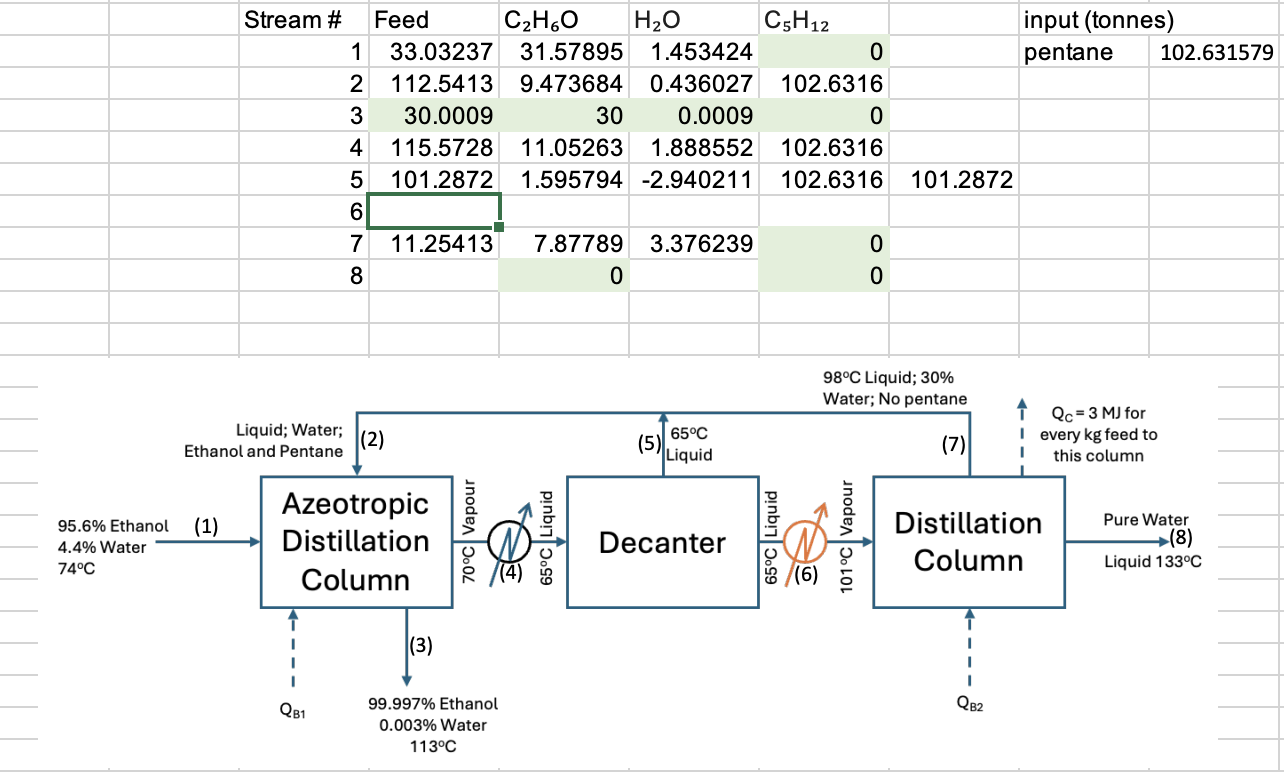
Conduct a detailed mass and energy balance for an azeotropic distillation plant producing 3 0 tonnes of pure ethanol every day from a water -
Conduct a detailed mass and energy balance for an azeotropic distillation plant producing tonnes of pure ethanol every day from a waterethanol feed containing approximately ethanol and water by weight.
Project Description
Distillation is a fundamental separation technique extensively utilized in the chemical and pharmaceutical industries to separate liquid mixtures based on their boiling points. Azeotropic distillation is specifically employed to separate azeotropic mixtures, such as the waterethanol system, which is a common binary mixture that forms an azeotrope. The waterethanol system forms a positive azeotrope, which means that the mixture has a higher boiling point than either pure water or pure ethanol. The azeotrope typically contains approximately ethanol and water by weight and boils at a constant temperature of
Azeotropic distillation of the waterethanol mixture involves adding an entrainer, such as benzene, pentane, or cyclohexane, to modify the relative volatility of the components and facilitate their separation. The entrainer forms a new azeotrope with one of the components, allowing for the breaking of the original azeotrope.
Below is a block diagram of the process:
Use Excel flowsheet to conduct detailed mass and energy balance of the plant. Assume the entire system to be operating at bars. Azeotropic separation requires pentane to fed at to times the amount of ethanol handled in the column in weight Undertake sensitivity studies on important calculated values as the function of the input values. Also conduct a dynamic study on the decanter because of disturbance in the input stream.
State all assumption appropriately, and collect necessary physical data from any open and reliable source.this is a mass balance i am currently trying to complete. it is for the azeotropic seperation process of ethanol and water using pentane. the green boxes are values that are not allowed to be changed. in addition to the percentage values given in the block diagram, assume that pentane is fed into the system at times the amount of ethanol handled in the column in weight the entire process is also assumed to produce tonnes of pure ethanol every day. Create more assumptions for the process in order to fill in and correct the spreadsheet values so that the entire process makes sense. Make sure to state clearly all assumptions used. The assumptions to be made that i havent already stated is the ratio for the stream going into the distillation column from the recycle stream and ratios for streams you may also change the amount of pentane to be fed into the system as long as it is between times the amount of ethanol handled in the column in weight
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


