Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Clark's Coffee Supply - Single and dual rate service department cost allocation Clark Griswold, owner and CEO of Clark's Coffee Supply, recently hired a
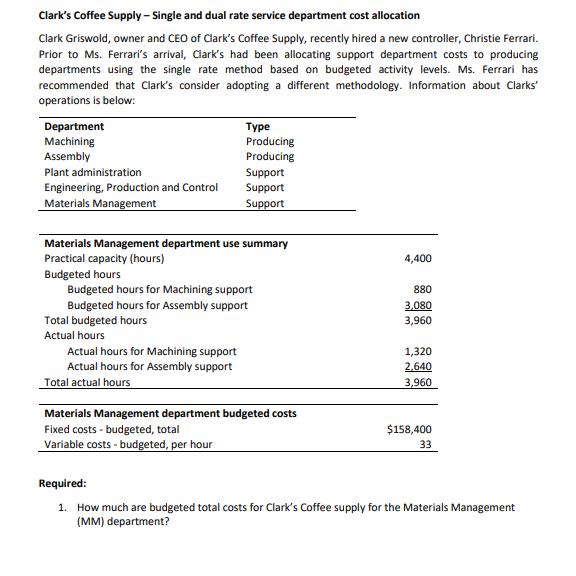

Clark's Coffee Supply - Single and dual rate service department cost allocation Clark Griswold, owner and CEO of Clark's Coffee Supply, recently hired a new controller, Christie Ferrari. Prior to Ms. Ferrari's arrival, Clark's had been allocating support department costs to producing departments using the single rate method based on budgeted activity levels. Ms. Ferrari has recommended that Clark's consider adopting a different methodology. Information about Clarks' operations is below: Department Machining Assembly Plant administration Engineering, Production and Control Materials Management Materials Management department use summary Practical capacity (hours) Budgeted hours Total budgeted hours Actual hours Type Producing Producing Budgeted hours for Machining support Budgeted hours for Assembly support Actual hours for Machining support Actual hours for Assembly support Support Support Support Total actual hours Materials Management department budgeted costs Fixed costs - budgeted, total Variable costs - budgeted, per hour 4,400 880 3,080 3,960 1,320 2,640 3,960 $158,400 33 Required: 1. How much are budgeted total costs for Clark's Coffee supply for the Materials Management (MM) department? 2. Compute the MM department support costs allocated to the Machining and Assembly departments under the current system (the single rate method using budgeted activity levels). 3. Compute the MM department support costs allocated to the Machining and Assembly departments using the dual rate method and budgeted activity levels.
Step by Step Solution
★★★★★
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


