Question
Consider a 0.25-kg piece of ice starting at -23C. Hint a. How much heat transfer is necessary to raise the temperature of the ice
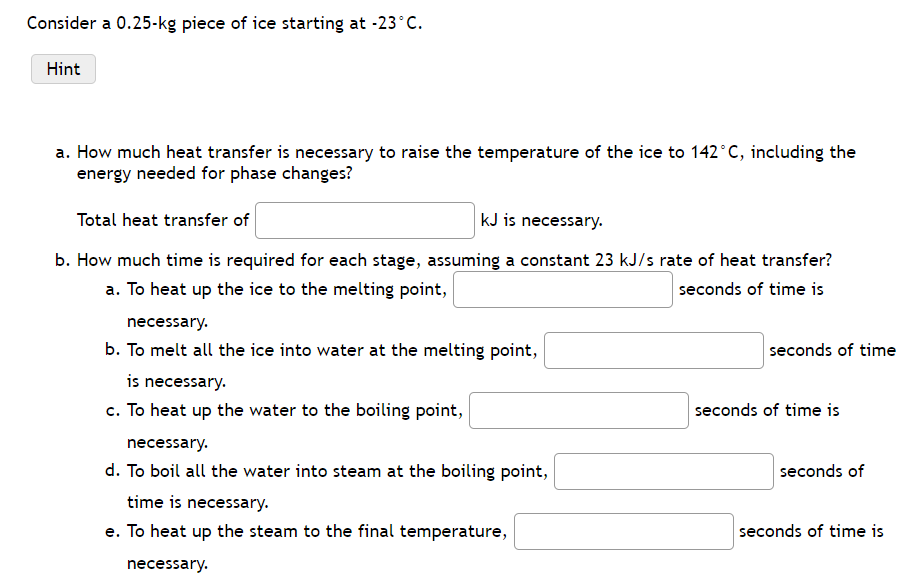
Consider a 0.25-kg piece of ice starting at -23C. Hint a. How much heat transfer is necessary to raise the temperature of the ice to 142C, including the energy needed for phase changes? Total heat transfer of KJ is necessary. b. How much time is required for each stage, assuming a constant 23 kJ/s rate of heat transfer? a. To heat up the ice to the melting point, necessary. seconds of time is b. To melt all the ice into water at the melting point, is necessary. c. To heat up the water to the boiling point, necessary. d. To boil all the water into steam at the boiling point, time is necessary. e. To heat up the steam to the final temperature, necessary. seconds of time seconds of time is seconds of seconds of time is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics
Authors: OpenStax
2nd Edition
171147083X, 978-1711470832
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



