Question
Consider a Carnot-cycle heat pump with R-134a as the working fluid. Heat is absorbed into the R- 134a at 0 C, during which process
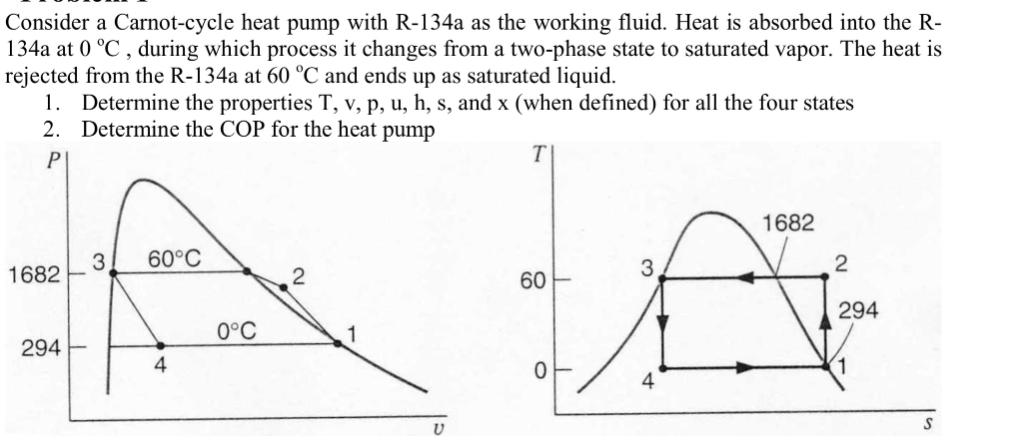
Consider a Carnot-cycle heat pump with R-134a as the working fluid. Heat is absorbed into the R- 134a at 0 C, during which process it changes from a two-phase state to saturated vapor. The heat is rejected from the R-134a at 60 C and ends up as saturated liquid. 1. Determine the properties T, v, p, u, h, s, and x (when defined) for all the four states 2. Determine the COP for the heat pump P 1682 294 3 60C 4 0C 2 U 60 0 3 4 1682 2 294
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



