Question
= Consider a deuterium atom (composed of a nucleus of spin 1 and an electron). The electronic angular momentum is J = L+S, where
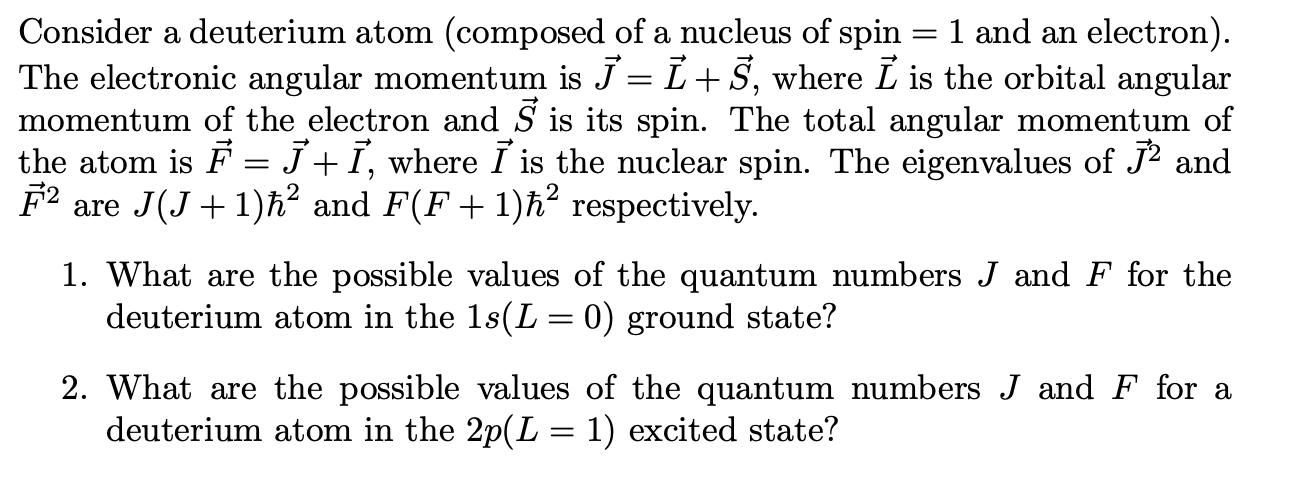
= Consider a deuterium atom (composed of a nucleus of spin 1 and an electron). The electronic angular momentum is J = L+S, where I is the orbital angular momentum of the electron and 5 is its spin. The total angular momentum of the atom is = J + , where I is the nuclear spin. The eigenvalues of J and F2 are J(J+1) and F(F+1) respectively. 1. What are the possible values of the quantum numbers J and F for the deuterium atom in the 1s(L = 0) ground state? 2. What are the possible values of the quantum numbers J and F for a deuterium atom in the 2p(L = 1) excited state?
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
1 In the ground state of a deuterium atom the electron occupies the 1s orbital with L 0 Since the el...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantum Chemistry
Authors: Ira N. Levine
7th edition
321803450, 978-0321803450
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



