Question
Consider a distillation column operating at a continuous steady state. The distillation column is seperating methanol & water at a pressure of 1 atm. This
Consider a distillation column operating at a continuous steady state. The distillation column is seperating methanol & water at a pressure of 1 atm. This operation uses an open system instead of a heat exchanger in the reboiler.
The stream used to heat up the steam is composed of ONLY saturated vapor & is fed into the bottom of column.
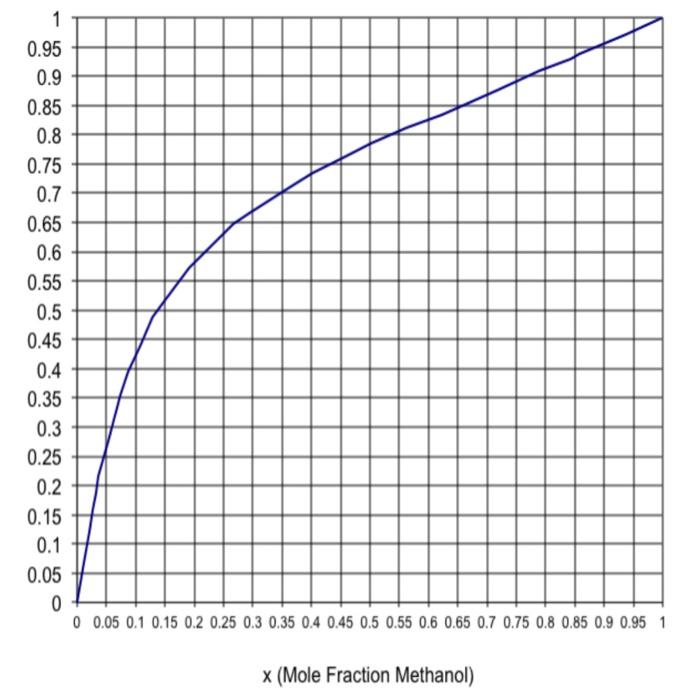
The McCabe-Thiele diagram is provided below. Please use it to solve this problem graphically.
The feed is composed of 60% methanol & 40% water by mol. The feed is operating at a rate of 100 kmol/h & a temperature of 40C.
The distillate is 99% methanol by mol. & the bottoms product is 2% methanol by mol (This insights that both the total condenser & the last equilibrium stage at the bottom of column as saturated liquids). Assume CMO (Constant Molal Overflow) rules apply here. If L/D = 2.3 & q = 1.0668 answer the following using the plot provided.
- Find the slope of the feed line (q-line) equation.
- Find the slope of the top-operating line (L/D) equation.
- Find the intercept of the top-operating line equation.
- Find the optimum feed stage location (find which stage assuming you are stepping from top to bottom)
- Find the total number of stages (Step from top to bottom)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


