Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a Joule-Thomson expansion (throttling) process. A gas at T, P flows in a well- insulated pipe and pass through a valve, with the
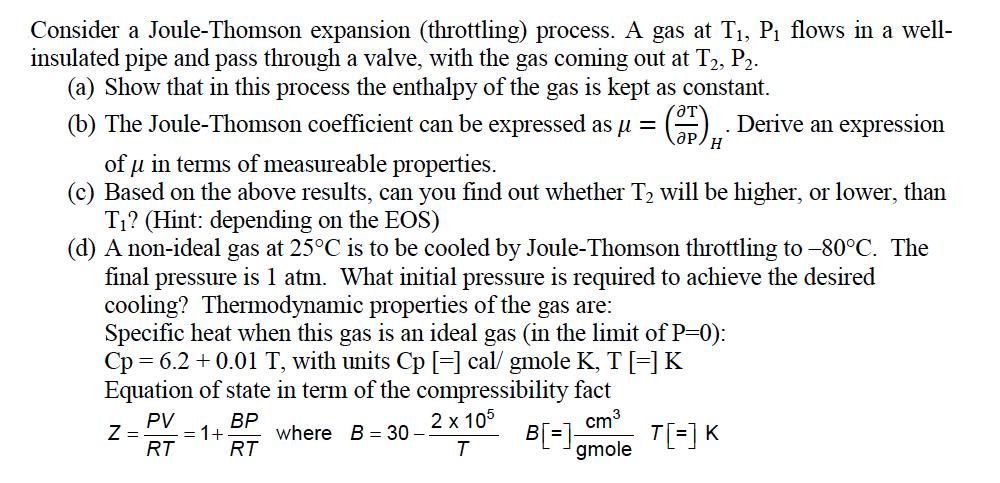
Consider a Joule-Thomson expansion (throttling) process. A gas at T, P flows in a well- insulated pipe and pass through a valve, with the gas coming out at T2, P2. (a) Show that in this process the enthalpy of the gas is kept as constant. (b) The Joule-Thomson coefficient can be expressed as = (3). Derive an expression H of u in terms of measureable properties. (c) Based on the above results, can you find out whether T will be higher, or lower, than T? (Hint: depending on the EOS) (d) A non-ideal gas at 25C is to be cooled by Joule-Thomson throttling to -80C. The final pressure is 1 atm. What initial pressure is required to achieve the desired cooling? Thermodynamic properties of the gas are: Specific heat when this gas is an ideal gas (in the limit of P=0): Cp = 6.2 +0.01 T, with units Cp [=] cal/gmole K, T[=] K Equation of state in term of the compressibility fact 2 x 105 cm PV Z = = 1+ RT BP RT B[=] T[=] K T gmole where B = 30- -
Step by Step Solution
★★★★★
3.40 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
a soring a throttling process a change in temperature a series of experiments takes place ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


