Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a potassium bromide (KBr) crystal with rocksalt (NaCl) structure. Draw a 2D representation of a color center and label all species in Krger-Vink

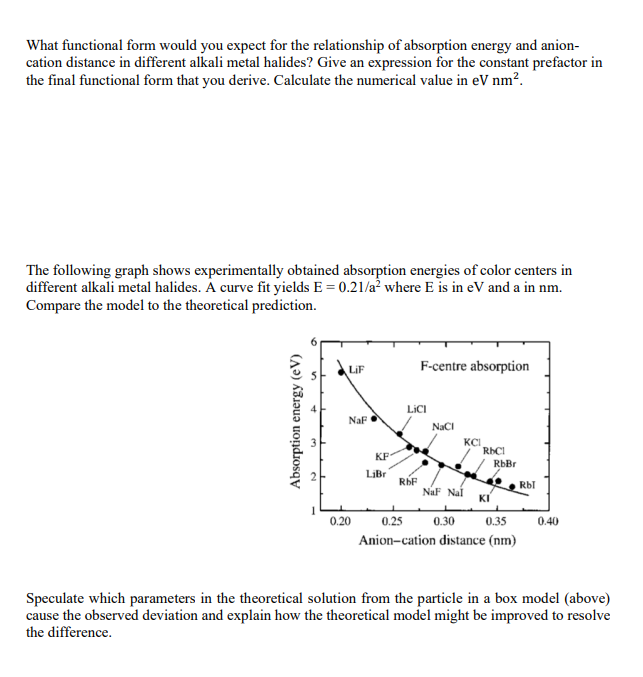
Consider a potassium bromide (KBr) crystal with rocksalt (NaCl) structure. Draw a 2D representation of a color center and label all species in Krger-Vink notation. How are color centers formed? Calculate the configurational entropy of ny = 1 x 1019 color centers in one mole potassium bromide. Show explicitly how Stirling's approximation is used to solve the problem. The particle in the box (PIB) model can be used to approximately describe color centers. The derivation starts from the time-independent Schrdinger equation: 7 = {0 Y = EY, = + with = - V and = 0 inside the box Derive an expression for the energy. 2m co else What are the values of nx, ny and n for the ground and first excited state leading to the characteristic absorption of a color center? What is the energy difference between the two states? What functional form would you expect for the relationship of absorption energy and anion- cation distance in different alkali metal halides? Give an expression for the constant prefactor in the final functional form that you derive. Calculate the numerical value in eV nm. The following graph shows experimentally obtained absorption energies of color centers in different alkali metal halides. A curve fit yields E = 0.21/a where E is in eV and a in nm. Compare the model to the theoretical prediction. Absorption energy (eV) LiF F-centre absorption LICI NaF NaCl KCI KF- RbCl RbBr LiBr RbF Rbl NaF Nal KI 0.20 0.25 0.30 0.35 0.40 Anion-cation distance (nm) Speculate which parameters in the theoretical solution from the particle in a box model (above) cause the observed deviation and explain how the theoretical model might be improved to resolve the difference.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


