Question
Consider a reactor that is filled with H2O at 240C and 4.2 MPa and is protected by a containment dome as shown in Fig. 3.
Consider a reactor that is filled with H2O at 240C and 4.2 MPa and is protected by a containment dome as shown in Fig. 3. The volume of the reactor is 5 m3 . The dome is insulated and is vacuum initially. An accident happens and the water inside the reactor starts to leak and eventually fills the entire containment dome. Hint: Insulated wall implies there is no heat transfer. (a) Estimate the minimum volume of the dome such that the final pressure does not exceed 200 kPa, which is the pressure limit of this dome. (b) What is the final temperature of H2O when it fills up the dome? (c) Draw a process curve on a T-v diagram indicating the initial state on its isobar line and the final state on its isobar line and relative to the saturated liquid and saturated vapour curve.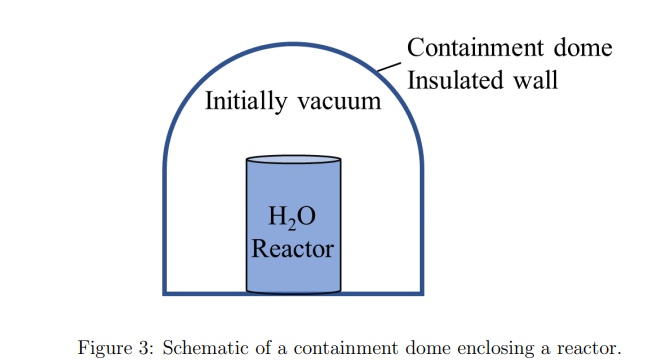
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


