Question
Consider a spherical catalyst pellet of radius R. Assume that the transport of the product is by diffusion, with diffusivity D, and that the transport
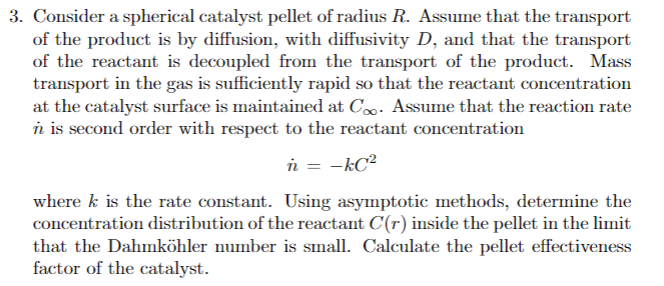
Consider a spherical catalyst pellet of radius R. Assume that the transport of the product is by diffusion, with diffusivity D, and that the transport of the reactant is decoupled from the transport of the product. Mass transport in the gas is sufficiently rapid so that the reactant concentration at the catalyst surface is maintained at C. Assume that the reaction rate n is second order with respect to the reactant concentration n = kC2 where k is the rate constant. Using asymptotic methods, determine the concentration distribution of the reactant C(r) inside the pellet in the limit that the Dahmk ohler number is small. Calculate the pellet effectiveness factor of the catalyst.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


