Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a system of 8 diatomic molecules with a total rotational system energy E = 8hcB and no other thermally accessible energy modes. The
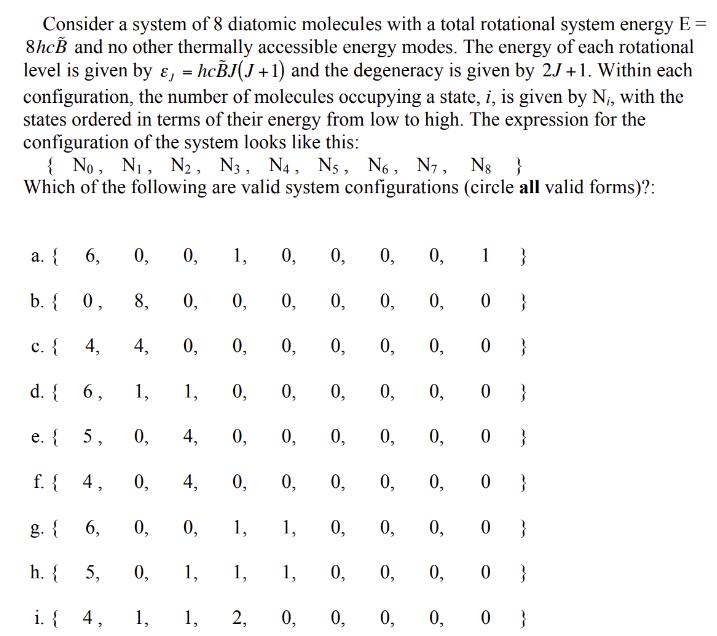
Consider a system of 8 diatomic molecules with a total rotational system energy E = 8hcB and no other thermally accessible energy modes. The energy of each rotational level is given by &, = hcBJ(J+1) and the degeneracy is given by 2J+1. Within each configuration, the number of molecules occupying a state, i, is given by N, with the states ordered in terms of their energy from low to high. The expression for the configuration of the system looks like this: { No, N, N2, N3, N4, N, N6, N, N8 } Which of the following are valid system configurations (circle all valid forms)?: a. { b. { c. { d. { e. { f. { a 6, 0. 6, 4, 4, 1, 5, 0, 0, 1, 0, 8, h. { 5, 4, 0, i. { 4, 0, 6, 0, 0, 1, 0, 0, 0, 0, 1, 0, 0, 4, 0, 0, 0, 0, 4, 0, 0, 0, 1, 1, 1, 1, 1, 1, 2, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0 0, 0, 0, 0, 0 } 0, 0, 0, 0, 0, 0, 0, 1} 0, 0, 0, 0, 0, 0 } 0, 0 } 0} 0 } 0 } 0 } } 0, 0
Step by Step Solution
There are 3 Steps involved in it
Step: 1
We must confirm that the total energy of the system equals the supplied expression and that the tota...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


