Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a two energy level system like we approached in class and HW, where the two energy level are A = 0 and B
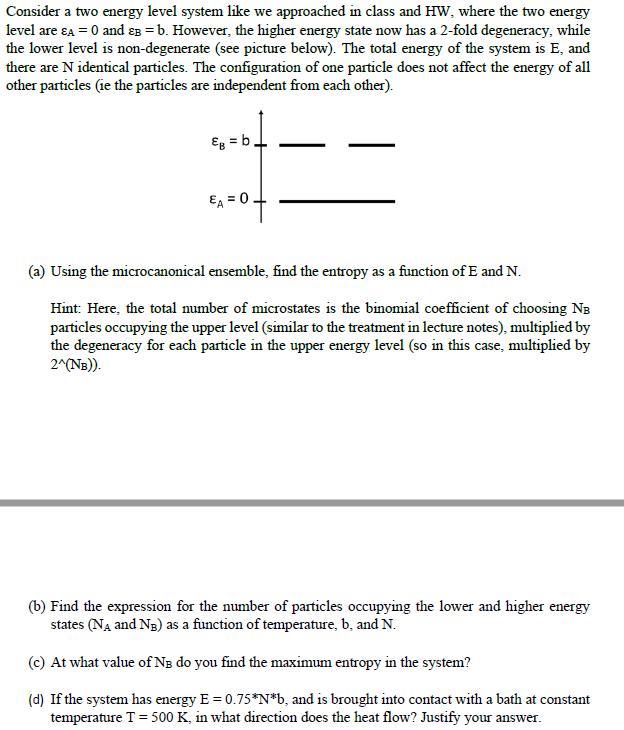
Consider a two energy level system like we approached in class and HW, where the two energy level are A = 0 and B = b. However, the higher energy state now has a 2-fold degeneracy, while the lower level is non-degenerate (see picture below). The total energy of the system is E, and there are N identical particles. The configuration of one particle does not affect the energy of all other particles (ie the particles are independent from each other). EA = 0. (a) Using the microcanonical ensemble, find the entropy as a function of E and N. Hint: Here, the total number of microstates is the binomial coefficient of choosing NB particles occupying the upper level (similar to the treatment in lecture notes), multiplied by the degeneracy for each particle in the upper energy level (so in this case, multiplied by 2^(NB)). (b) Find the expression for the number of particles occupying the lower and higher energy states (N and NB) as a function of temperature, b, and N. (c) At what value of N do you find the maximum entropy in the system? (d) If the system has energy E = 0.75*N*b, and is brought into contact with a bath at constant temperature T = 500 K, in what direction does the heat flow? Justify your answer.
Step by Step Solution
★★★★★
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
a Entropy as a function of E and N The entropy S is given by Skln Where N B N 2 N B is the to...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


