Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider methanol (T=512.6 K, P= 8.096 MPa) described by the van der Waals equation of state, which has constants a = 0.946 Pa m6
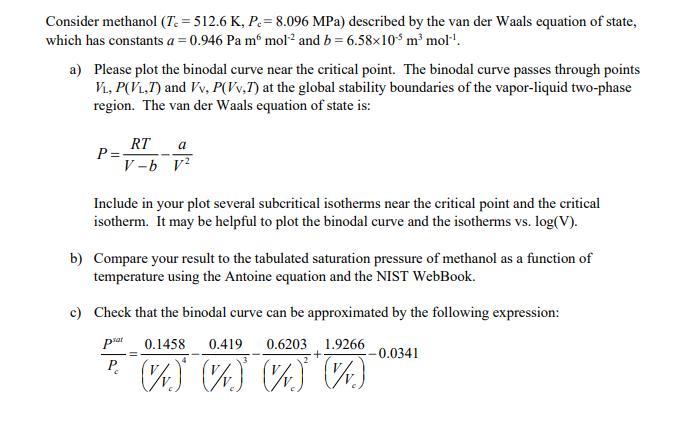
Consider methanol (T=512.6 K, P= 8.096 MPa) described by the van der Waals equation of state, which has constants a = 0.946 Pa m6 mol and b = 6.5810 m mol. a) Please plot the binodal curve near the critical point. The binodal curve passes through points VL, P(VL.T) and Vv, P(Vv,T) at the global stability boundaries of the vapor-liquid two-phase region. The van der Waals equation of state is: P= RT a V-b v2 Include in your plot several subcritical isotherms near the critical point and the critical isotherm. It may be helpful to plot the binodal curve and the isotherms vs. log(V). b) Compare your result to the tabulated saturation pressure of methanol as a function of temperature using the Antoine equation and the NIST WebBook. c) Check that the binodal curve can be approximated by the following expression: psat 0.1458 0.419 0.6203 1.9266 + P. V v ) V v ) V v ) (V) -0.0341
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


