Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider spills of 10 L of tetrachloroethene (also known as perchloroethene or PCE). What is the maximum concentration of PCE (in g/m3 ) that you
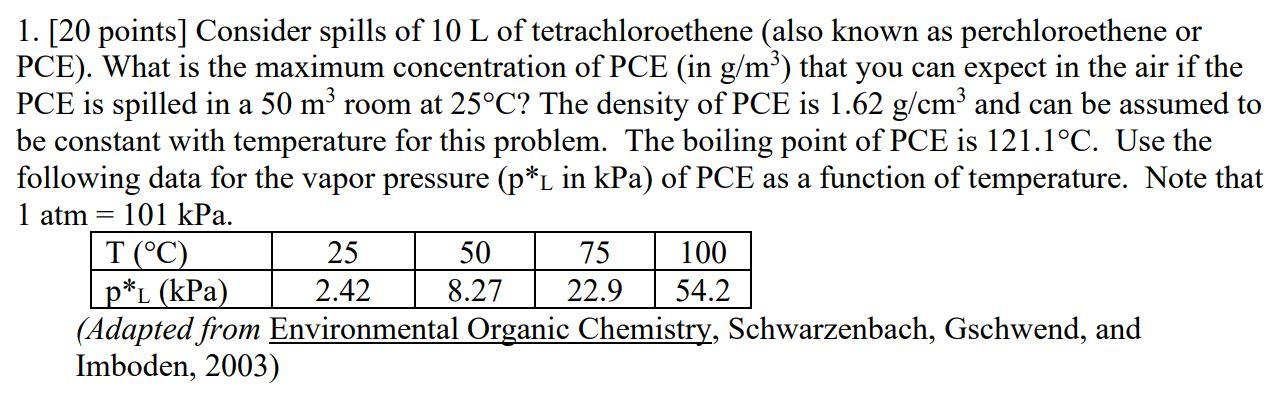 Consider spills of 10 L of tetrachloroethene (also known as perchloroethene or PCE). What is the maximum concentration of PCE (in g/m3 ) that you can expect in the air if the PCE is spilled in a 50 m3 room at 25C? The density of PCE is 1.62 g/cm3 and can be assumed to be constant with temperature for this problem. The boiling point of PCE is 121.1C. Use the following data for the vapor pressure (p*L in kPa) of PCE as a function of temperature. Note that 1 atm = 101 kPa.
Consider spills of 10 L of tetrachloroethene (also known as perchloroethene or PCE). What is the maximum concentration of PCE (in g/m3 ) that you can expect in the air if the PCE is spilled in a 50 m3 room at 25C? The density of PCE is 1.62 g/cm3 and can be assumed to be constant with temperature for this problem. The boiling point of PCE is 121.1C. Use the following data for the vapor pressure (p*L in kPa) of PCE as a function of temperature. Note that 1 atm = 101 kPa.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


