Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the absorption of component ( A ) of a gaseous mixture with an inert ( I ) by a phase liquid containing a reactive
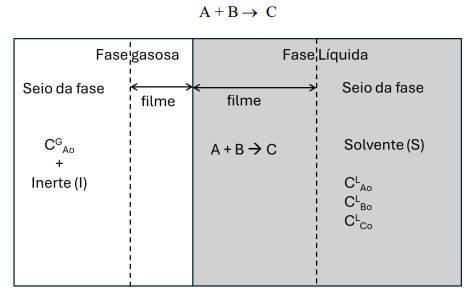
Consider the absorption of component A of a gaseous mixture with an inert I by a phase liquid containing a reactive species B and a solvent S Component A reacts instantly with component B according to the following reaction: The Process occurs in an established regime, temperature is constant and the gas and liquid phasescan be considered ideal. Solvent S reactant B and product C are nonvolatile and their transfer to the gas phase can be considered null. The inert compound in the gas phase is not soluble in solvent S
a Assume that the concentrations within each phase are known and sketch the profiles of concentration for A and I in the gas phase; and the profiles of A B and C in the liquid phase. Also consider
that the concentration of the solvent is practically constant throughout the liquid phase.
b Write the equations for mass transfer in each phase assuming that the diffusion coefficients are known and constant.
c Write the boundary conditions necessary to calculate the absorption flow of the component A
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


