Answered step by step
Verified Expert Solution
Question
1 Approved Answer
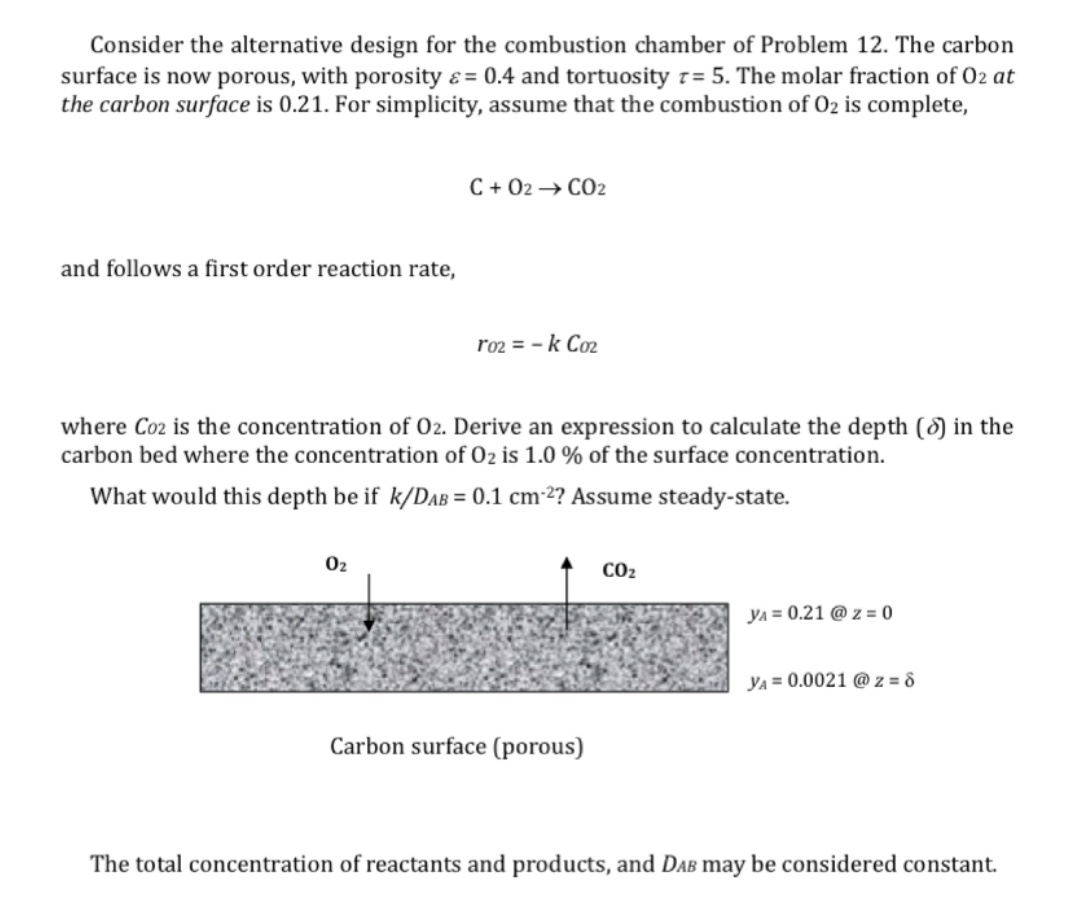
Consider the alternative design for the combustion chamber of Problem 1 2 . The carbon surface is now porous, with porosity = 0 . 4
Consider the alternative design for the combustion chamber of Problem The carbon surface is now porous, with porosity and tortuosity The molar fraction of at the carbon surface is For simplicity, assume that the combustion of is complete,
and follows a first order reaction rate,
where is the concentration of Derive an expression to calculate the depth in the carbon bed where the concentration of is of the surface concentration.
What would this depth be if Assume steadystate.
@
@
Carbon surface porous
The total concentration of reactants and products, and may be considered constant.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


