Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the chemical potentials of the species under the given conditions: i. A solution of n-heptane (Hep) and n-octane (Oc) behaves ideally. Calculate the chemical
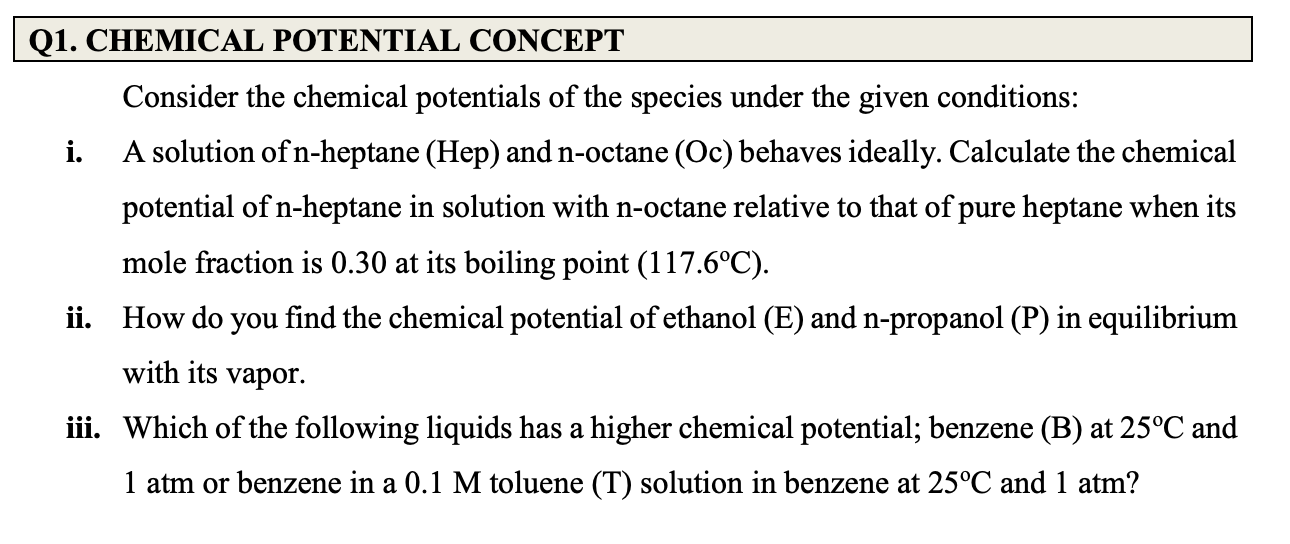 Consider the chemical potentials of the species under the given conditions: i. A solution of n-heptane (Hep) and n-octane (Oc) behaves ideally. Calculate the chemical potential of n-heptane in solution with n-octane relative to that of pure heptane when its mole fraction is 0.30 at its boiling point (117.6C). ii. How do you find the chemical potential of ethanol (E) and n-propanol (P) in equilibrium with its vapor. iii. Which of the following liquids has a higher chemical potential; benzene (B) at 25C and 1 atm or benzene in a 0.1M toluene (T) solution in benzene at 25C and 1atm
Consider the chemical potentials of the species under the given conditions: i. A solution of n-heptane (Hep) and n-octane (Oc) behaves ideally. Calculate the chemical potential of n-heptane in solution with n-octane relative to that of pure heptane when its mole fraction is 0.30 at its boiling point (117.6C). ii. How do you find the chemical potential of ethanol (E) and n-propanol (P) in equilibrium with its vapor. iii. Which of the following liquids has a higher chemical potential; benzene (B) at 25C and 1 atm or benzene in a 0.1M toluene (T) solution in benzene at 25C and 1atm Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


