Question
Consider the dehydrogenation of ethylene to produce acetylene 1. Estimate the equilibrium constant at 500 and 1000 K, assuming DH independent of temperature, using the
Consider the dehydrogenation of ethylene to produce acetylene
1. Estimate the equilibrium constant at 500 and 1000 K, assuming DH independent of temperature, using the following information
| C2H4 (g) | C2H2 (g) | H2(g) | |
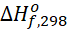 [J/mol] [J/mol] | 52 510 | 227 480 | 0 |
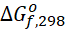 [J/mol] [J/mol] | 68 460 | 209 970 | 0 |
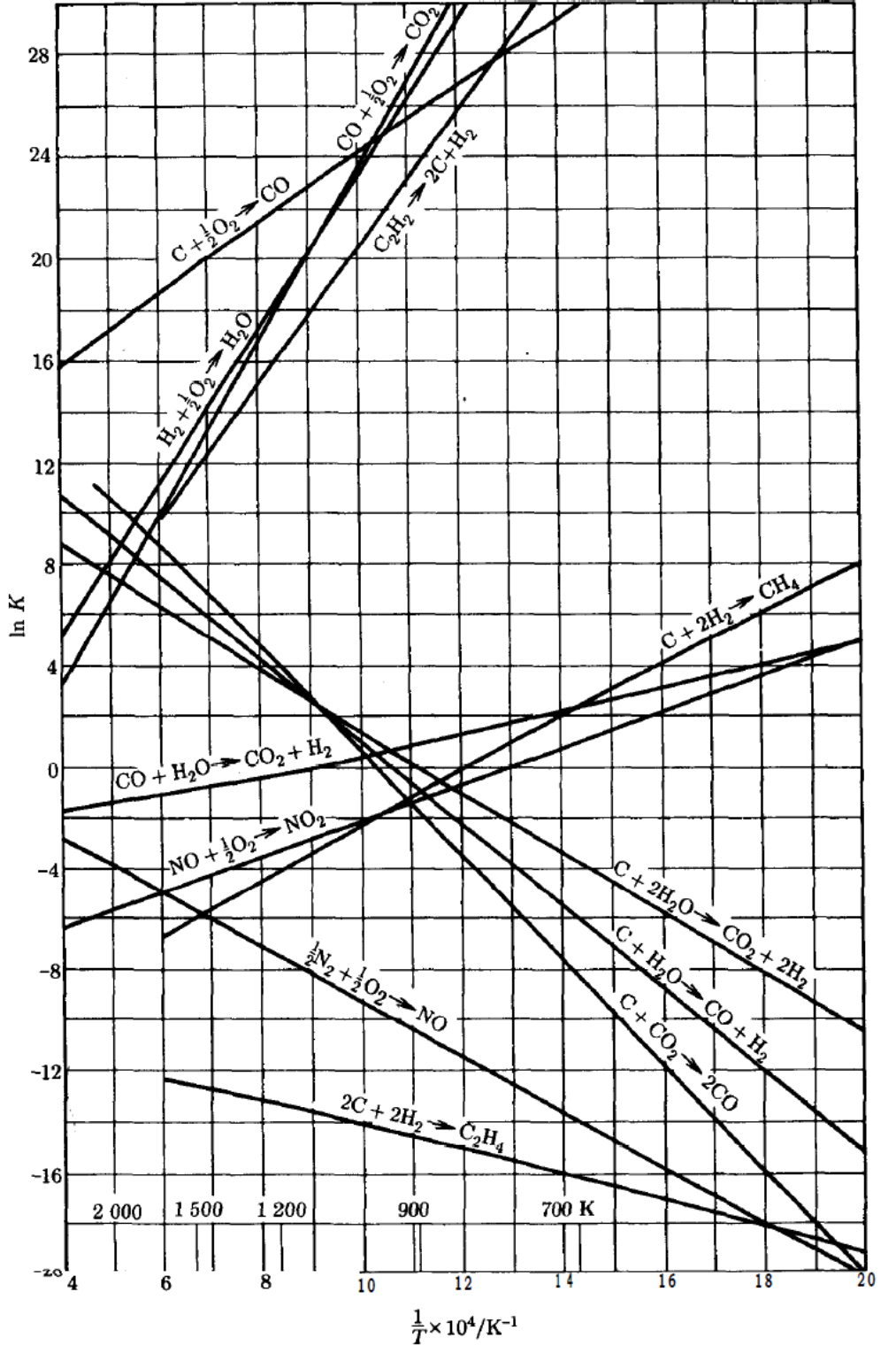
2. Estimate the equilibrium constant, at 500 and at 1000 K considering the full effect of temperature, using the graph ln K vs 1/T (see next page), compare with the results of the previous point and analyze.
3. If the purpose of the reaction is to obtain as much acetylene as possible, the process should be carried out:
a) high temperature and moderate pressure
b) high temperature and high pressure
c) moderate temperature and moderate pressure
d) moderate temperature and high pressure
explain your answer
Hf,298o Gf,298oStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


