Answered step by step
Verified Expert Solution
Question
1 Approved Answer
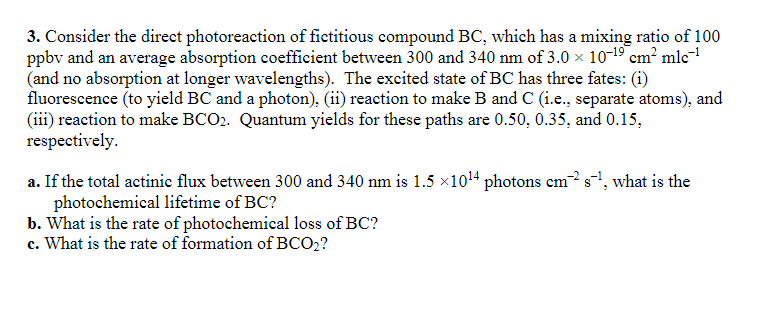
Consider the direct photoreaction of fictitious compound B C , which has a mixing ratio of 1 0 0 ppbv and an average absorption coefficient
Consider the direct photoreaction of fictitious compound which has a mixing ratio of
ppbv and an average absorption coefficient between and of
and no absorption at longer wavelengths The excited state of has three fates: i
fluorescence to yield and a photonii reaction to make and ie separate atoms and
iii reaction to make Quantum yields for these paths are and
respectively.
a If the total actinic flux between and is photons what is the
photochemical lifetime of
b What is the rate of photochemical loss of
c What is the rate of formation of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


