Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the following half redox .19 reaction involving NO3 at a concentration of 10^-5 M and ammonium at a concentration of 10^-3 M in groundwater



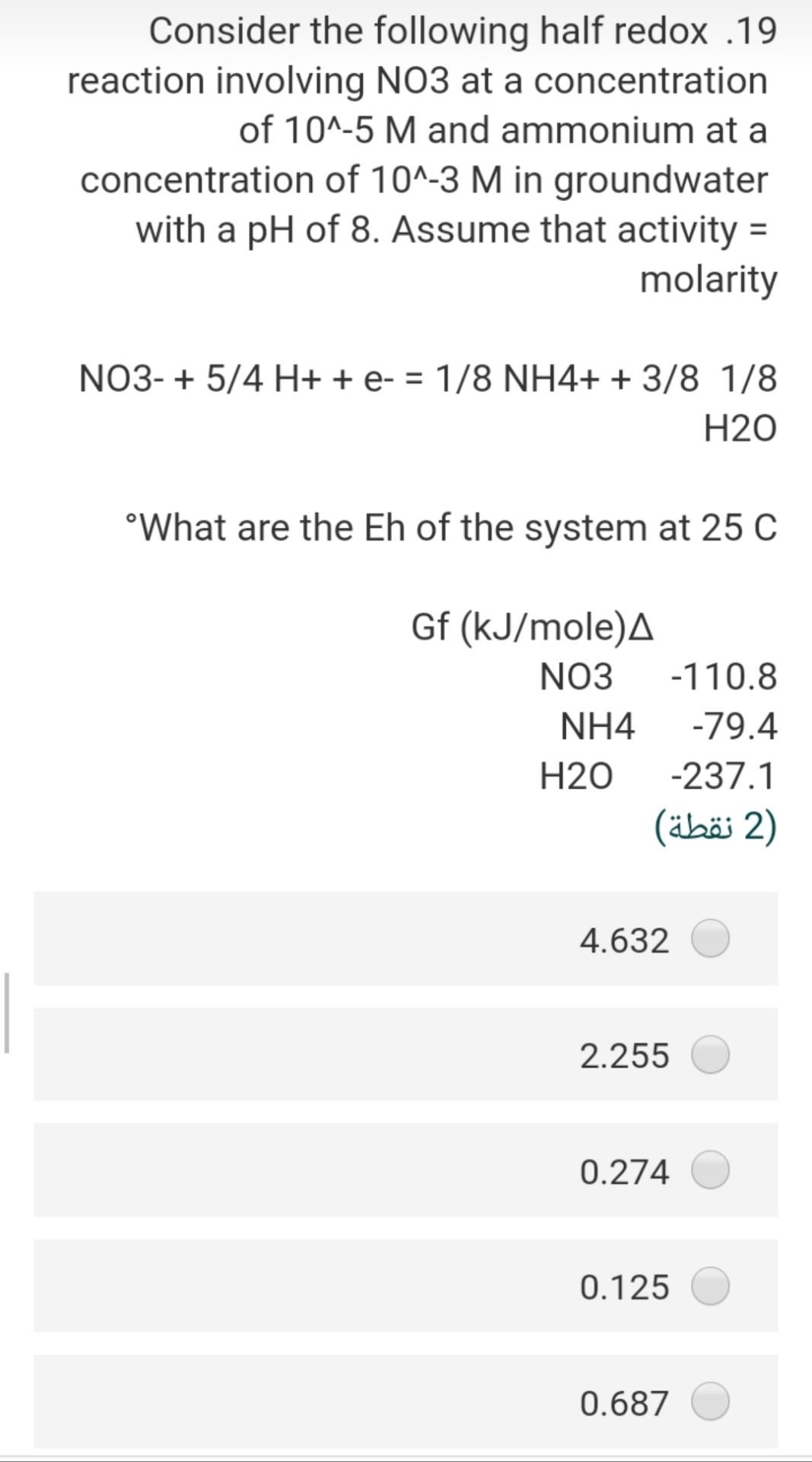

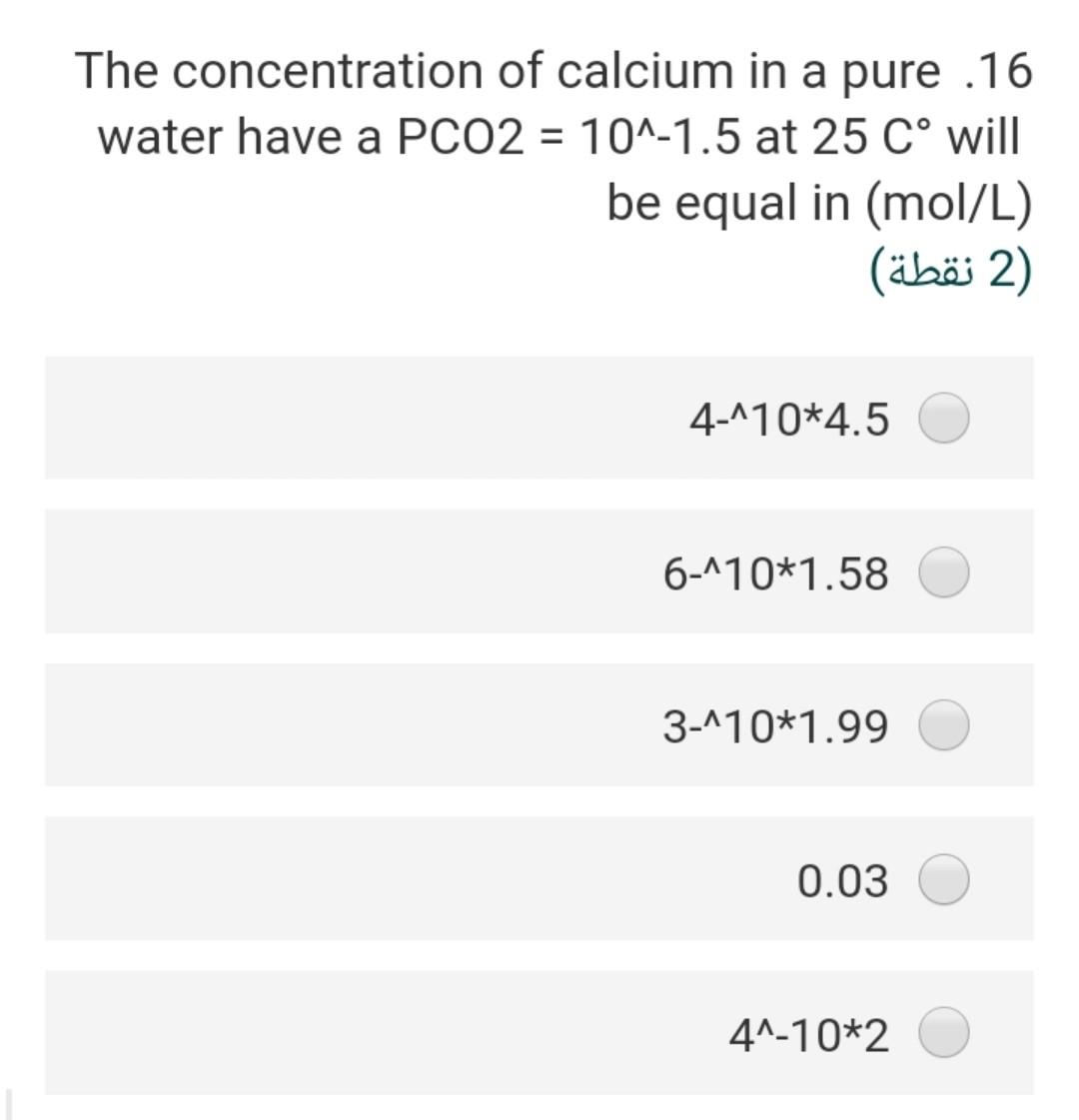

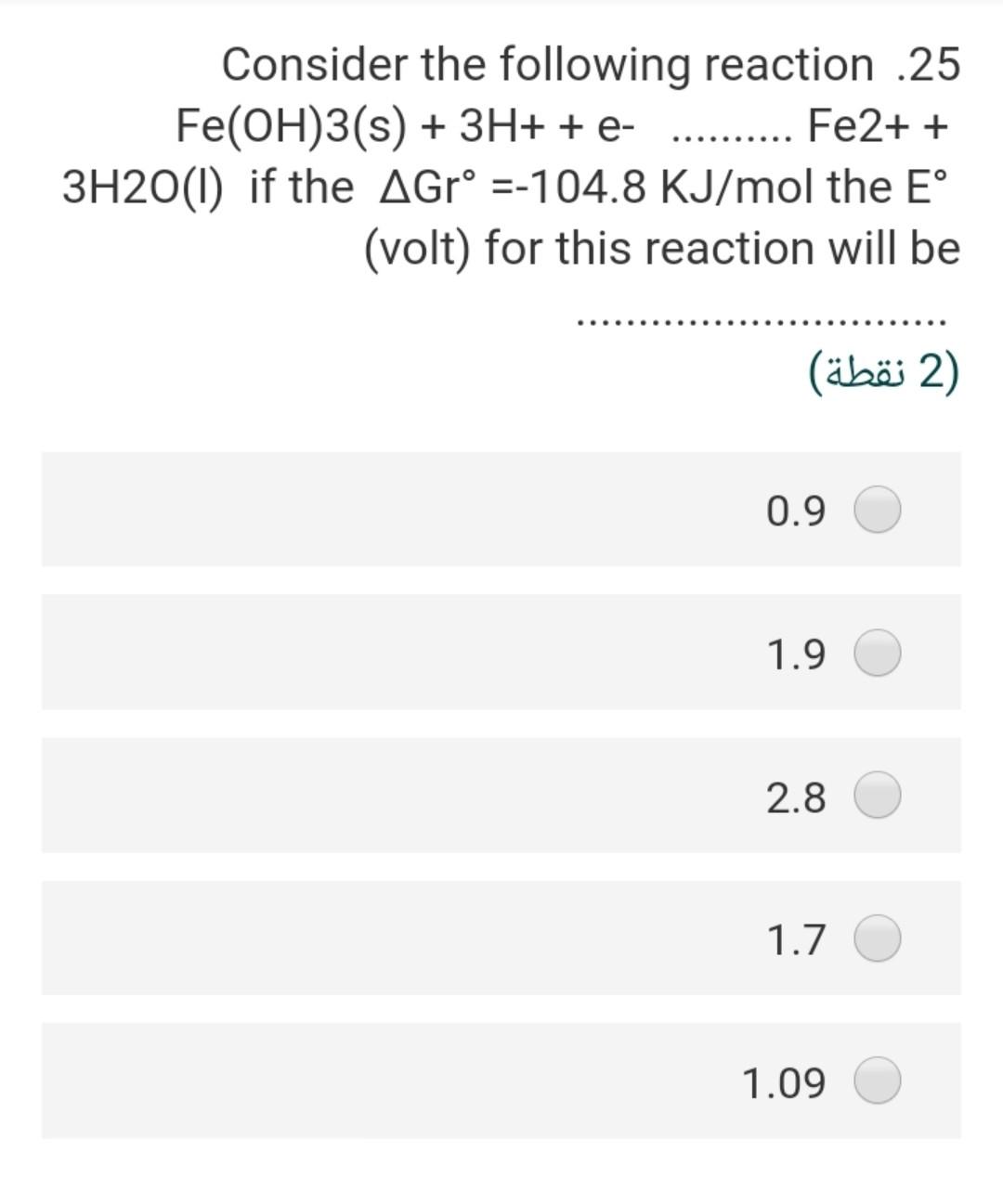

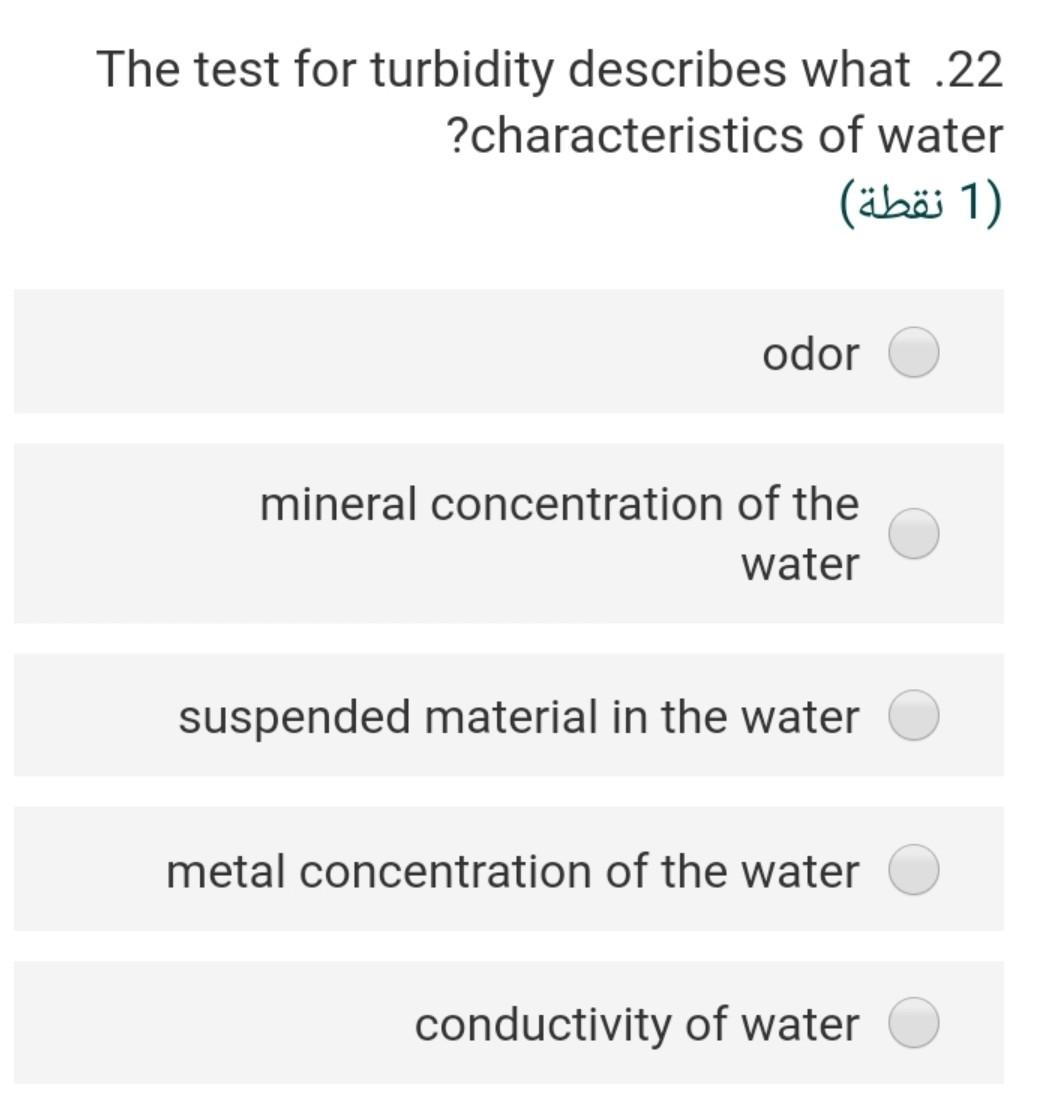

Consider the following half redox .19 reaction involving NO3 at a concentration of 10^-5 M and ammonium at a concentration of 10^-3 M in groundwater with a pH of 8. Assume that activity = molarity NO3- + 5/4 H+ + e- = 1/8 NH4+ + 3/8 1/8 H20 What are the Eh of the system at 25 C Gf (kJ/mole). NO3 -110.8 NH4 -79.4 H20 -237.1 (2 ) 4.632 2.255 0.274 0.125 Taking into consideration the following .12 :chemical reactions CO2(g) + H2O + H2CO3 pK=1.5 H2CO3 H + HCO pK= 6.4 HCO3 H + CO3 pK= 10.3 What would be the pH-value of rainwater, if it is in equilibrium with the PCO2 = 10^-3.5 in the atmosphere? (In the absence of substances such as SOX, NOX and NH3 in the air (2) 4.5 5.7 6.7 4.8 6.5 A water sample has a hardness of 560 .18 mg/l as CaCo3 and Mg content of 72 mg/l, the concentration of Ca in (mg/l) will ....... be (2) 5.6 106 518 7.8 218 Consider the following half redox .19 reaction involving No3 at a concentration of 10^-5 M and ammonium at a concentration of 10^-3 M in groundwater with a pH of 8. Assume that activity = molarity NO3- + 5/4 H+ + e- = 1/8 NH4+ + 3/8 1/8 H20 What are the Eh of the system at 25 C Gf (kJ/mole). NO3 -110.8 NH4 -79.4 H20 -237.1 2) (2 ) 4.632 2.255 0.274 0.125 0.687 The pH of 0.0027 molal solution of .26 H2CO3 is 2 (2 ) 5.60 3.45 4.48 6.45 7.80 The concentration of calcium in a pure .16 water have a PCO2 = 10^-1.5 at 25 C will be equal in (mol/L) 2) (2 ) 4-110*4.5 6-110*1.58 3-110*1.99 0.03 41-10*2 If the Eh of the groundwater sample 7 equal 0.35, the Pe of this water at 25C will be (2) 5.92 16.9 0.02 3.50 48.30 Consider the following reaction .25 Fe(OH)3(s) + 3H+ + e- ....... Fe2+ + 3H20(1) if the AGr =-104.8 KJ/mol the E (volt) for this reaction will be (2 ) 0.9 1.9 2.8 1.7 1.09 If the total alkalinity of water sample .21 was calculated by titration with initial pH of 7.2 to be 86.1 mg/L as CaCO3, the molarity of bicarbonate (M HCO3) is (2 ) x 10^-3 M 4.57 M 3-110x1.722 x 10^-5 M 2.552 x 10^-4 M 1.860 x 10^-5 M 8.672 The test for turbidity describes what .22 ?characteristics of water 1 (1 ) odor mineral concentration of the water suspended material in the water metal concentration of the water conductivity of water If the Eh of the groundwater sample 7 equal 0.35, the Pe of this water at 25C will be (2 (2 ) 5.92 16.9 0.02 3.50 48.30
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


