Answered step by step
Verified Expert Solution
Question
1 Approved Answer
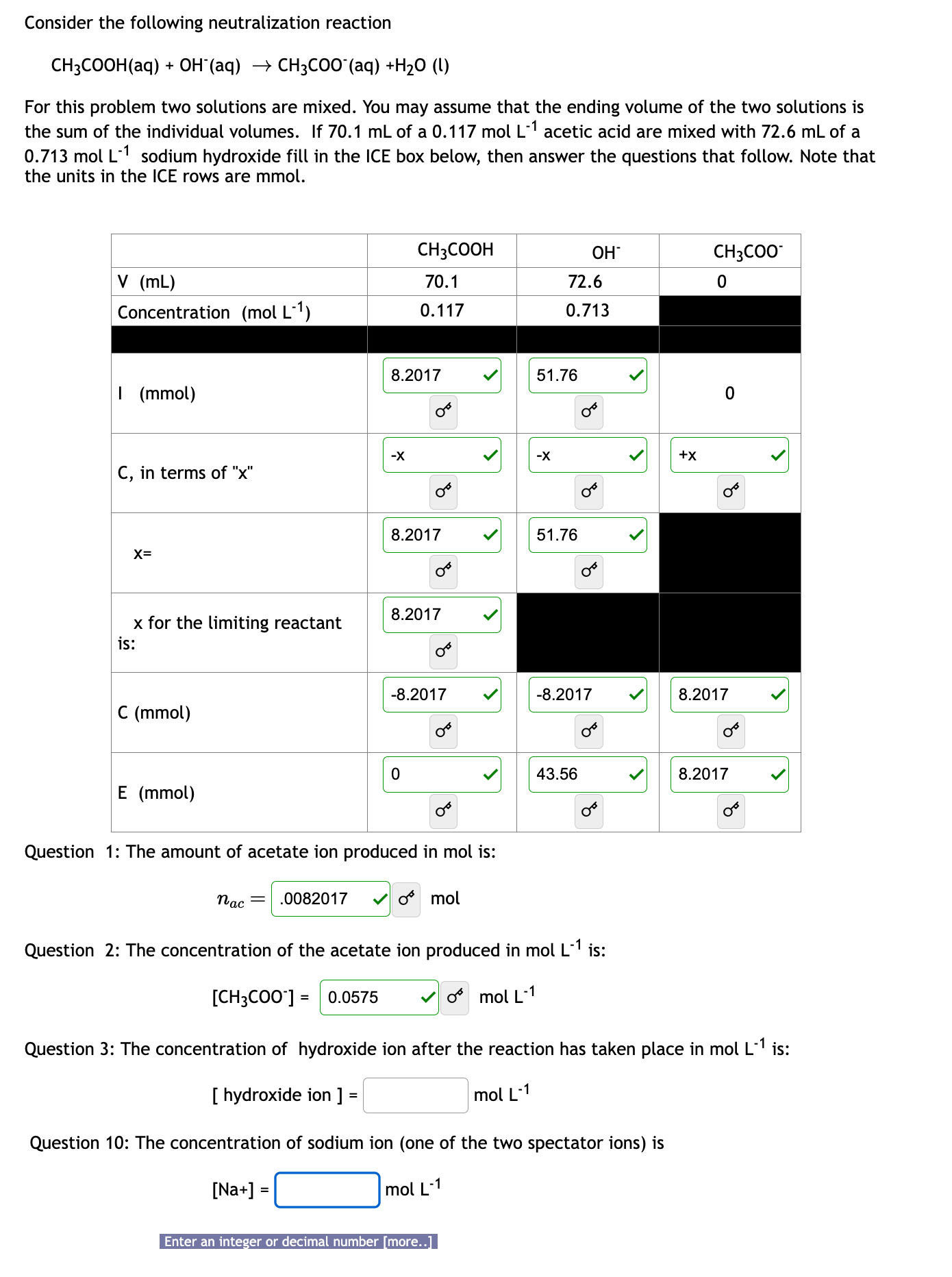
Consider the following neutralization reaction CH 3 COOH ( aq ) + OH - ( aq ) CH 3 COO - ( aq ) +
Consider the following neutralization reaction
CHCOOHaq OHaq
CHCOOaqHO l
For this problem two solutions are mixed. You may assume that the ending volume of the two solutions is the sum of the individual volumes. If mL of a mol L acetic acid are mixed with mL of a mol L sodium hydroxide fill in the ICE box below, then answer the questions that follow. Note that the units in the ICE rows are mmol.
For this problem two solutions are mixed. You may assume that the ending volume of the two solutions is
the sum of the individual volumes. If of acetic acid are mixed with of a
sodium hydroxide fill in the ICE box below, then answer the questions that follow. Note that
the units in the ICE rows are mmol.
Question : The amount of acetate ion produced in mol is:
mol
Question : The concentration of the acetate ion produced in is:
Question : The concentration of hydroxide ion after the reaction has taken place in is:
hydroxide ion
Question : The concentration of sodium ion one of the two spectator ions is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


