Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the following nuclear reaction: atomic mass (amu) of V-51 = 50.9440; atomic mass (amu) of Cr-52 = 51.9405 mass (amu) : n = 1.0087
Consider the following nuclear reaction: 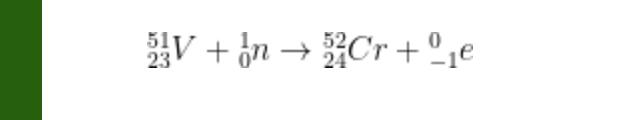
atomic mass (amu) of V-51 = 50.9440; atomic mass (amu) of Cr-52 = 51.9405 mass (amu) : n = 1.0087 Mass (amu): e = 0.0006 Answer the following questions:
The mass change per nuclide for this reaction: amu (to 4 decimal places)
The mass change per mole for this reaction: g (to 4 decimal places)
The energy released change per nuclide for this reaction: J (to 3 significant figures)
The energy released change per mole for this reaction: J (to 3 significant figures)
2351V+01n2452Cr+10eStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


