Answered step by step
Verified Expert Solution
Question
1 Approved Answer
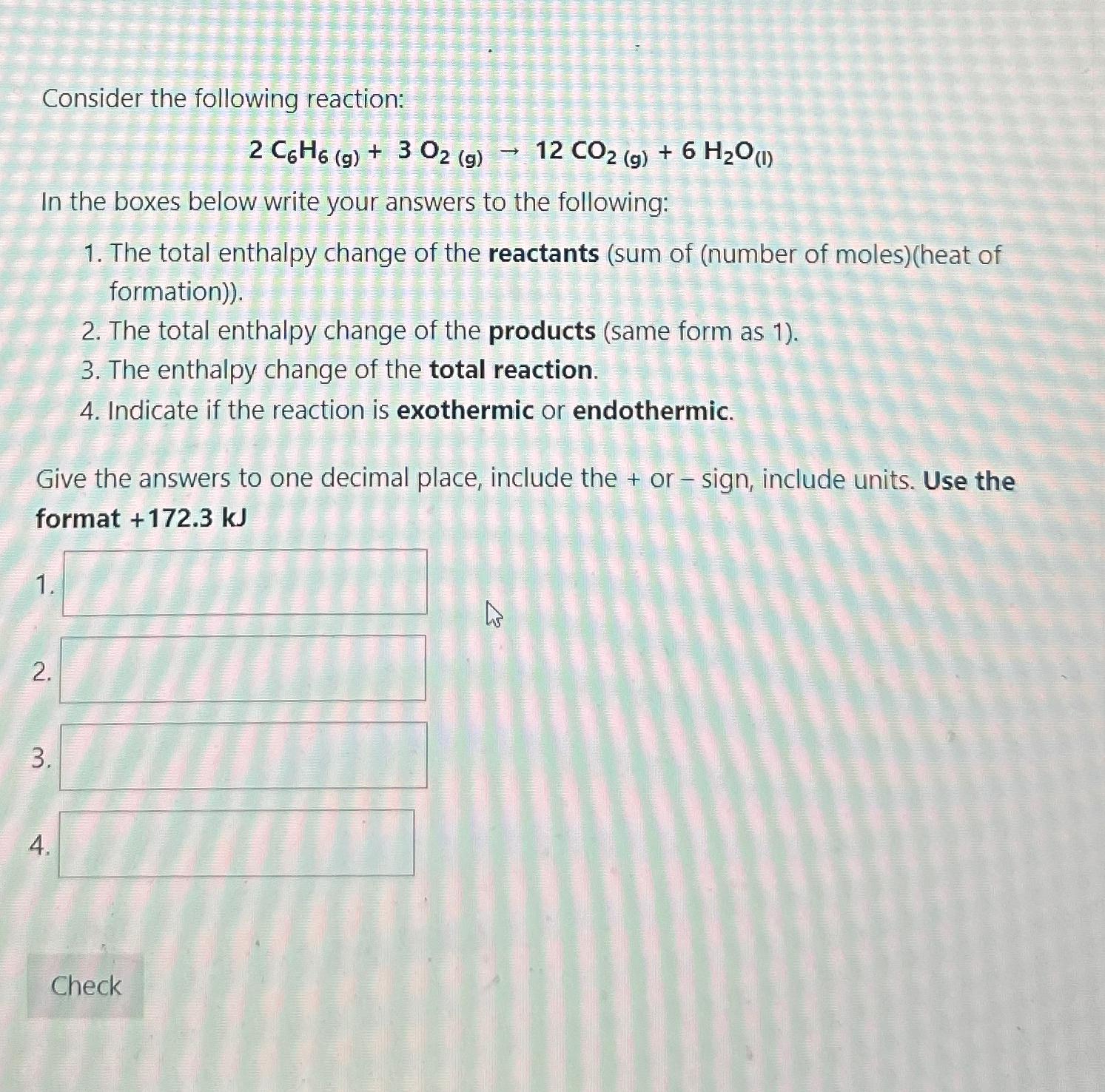
Consider the following reaction: 2 C 6 H 6 ( g ) + 3 O 2 ( g ) 1 2 C O 2 (
Consider the following reaction:
In the boxes below write your answers to the following:
The total enthalpy change of the reactants sum of number of molesheat of formation
The total enthalpy change of the products same form as
The enthalpy change of the total reaction.
Indicate if the reaction is exothermic or endothermic.
Give the answers to one decimal place, include the or sign, include units. Use the format
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


