Answered step by step
Verified Expert Solution
Question
1 Approved Answer
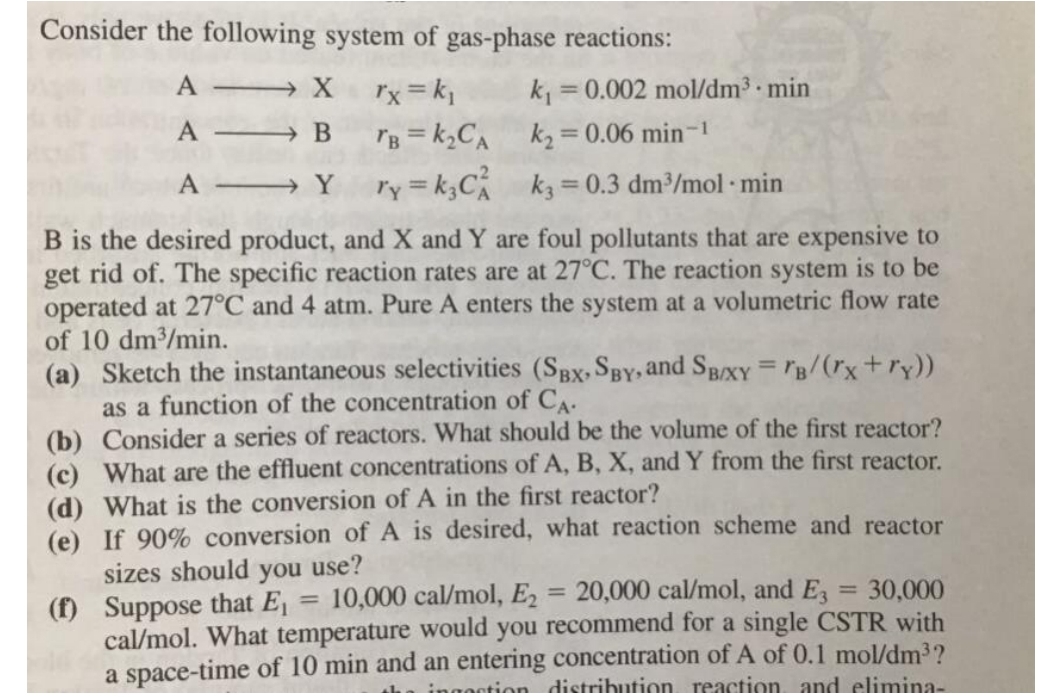
Consider the following system of gas - phase reactions: A X , r x = k 1 , k 1 = 0 . 0 0
Consider the following system of gasphase reactions:
A X
A B
A
is the desired product, and and are foul pollutants that are expensive to get rid of The specific reaction rates are at The reaction system is to be operated at and atm. Pure A enters the system at a volumetric flow rate of
a Sketch the instantaneous selectivities and : as a function of the concentration of
b Consider a series of reactors. What should be the volume of the first reactor?
c What are the effluent concentrations of A B X and Y from the first reactor.
d What is the conversion of in the first reactor?
e If conversion of is desired, what reaction scheme and reactor sizes should you use?
f Suppose that and What temperature would you recommend for a single CSTR with a spacetime of min and an entering concentration of of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


