Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the following T- xy diagram of ethanol(1)/toluene(2) and 1 atm. a) b) Identify the normal boiling point of species 1 and 2 as
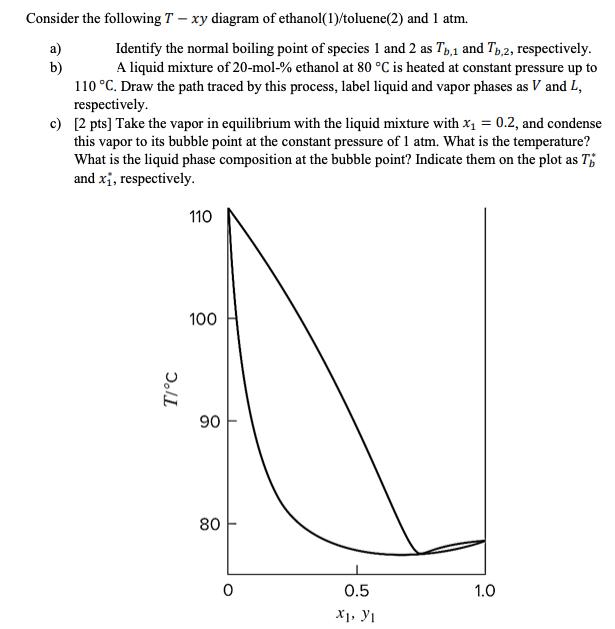
Consider the following T- xy diagram of ethanol(1)/toluene(2) and 1 atm. a) b) Identify the normal boiling point of species 1 and 2 as Tb,1 and Tb,2, respectively. A liquid mixture of 20-mol-% ethanol at 80 C is heated at constant pressure up to 110 C. Draw the path traced by this process, label liquid and vapor phases as V and L, respectively. c) [2 pts] Take the vapor in equilibrium with the liquid mixture with x = 0.2, and condense this vapor to its bubble point at the constant pressure of 1 atm. What is the temperature? What is the liquid phase composition at the bubble point? Indicate them on the plot as T and x, respectively. T/C 110 100 90 80 0 0.5 XI, YI 1.0
Step by Step Solution
★★★★★
3.40 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
Certainly Lets break down the problem step by step and construct a Txy diagram for the given ethanol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


