Question
Consider the following two gas-phase reactions: A+B C 2C D The equilibrium constants of the two reactions are given by, K, =- a a,
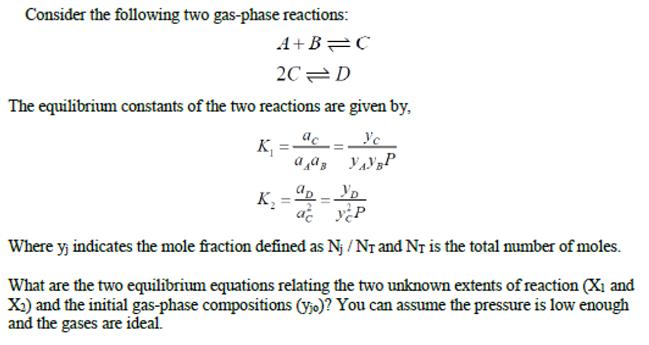
Consider the following two gas-phase reactions: A+B C 2C D The equilibrium constants of the two reactions are given by, K, =- a a, yaP %3D K. Where y, indicates the mole fraction defined as Nj / Nr and Nr is the total mumber of moles. What are the two equilibrium equations relating the two unknown extents of reaction (X1 and X2) and the initial gas-phase compositions (y;o)? You can assume the pressure is low enough and the gases are ideal.
Step by Step Solution
3.57 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Corporate Finance
Authors: Stephen Ross, Randolph Westerfield, Jeffrey Jaffe
10th edition
978-0077511388, 78034779, 9780077511340, 77511387, 9780078034770, 77511344, 978-0077861759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



