Question
Consider the following voltaic cell: Voltmeter Salt bridgo Zn Co 1 M Co2+ (a) in which direction do electrons flow in the external circuit?
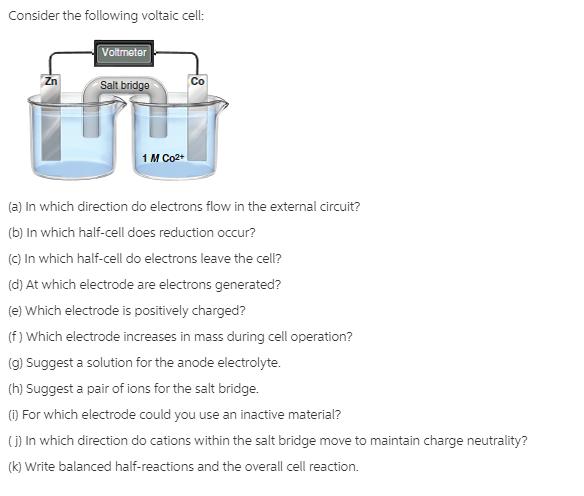
Consider the following voltaic cell: Voltmeter Salt bridgo Zn Co 1 M Co2+ (a) in which direction do electrons flow in the external circuit? (b) In which half-cell does reduction occur? (C) In which half-cell do electrons leave the cell? (d) At which electrode are electrons generated? (e) Which electrode is positively charged? (f ) Which electrode increases in mass during cell operation? (g) Suggest a solution for the anode electrolyte. (h) Suggest a pair of ions for the salt bridge. () For which electrode could you use an inactive material? ) In which direction do cations within the salt bridge move to maintain charge neutrality? (k) Write balanced half-reactions and the overall cell reaction.
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



