Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the gas container shown in Figure 3-4, the container sinking as a buffer or surge tank in a process. It is assumed that the
Consider the gas container shown in Figure 3-4, the container sinking as a buffer or surge tank in a process. It is assumed that the process takes place isothermally, at a temperature T, and that the flow through the outlet valve is expressed by 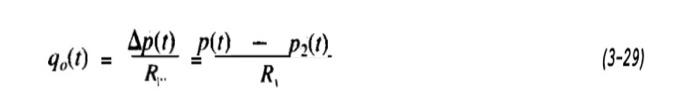
 1 equation, 2 unknowns (qo(t), m(t))
1 equation, 2 unknowns (qo(t), m(t))  2 equations, 3 unknowns (p(t))
2 equations, 3 unknowns (p(t))
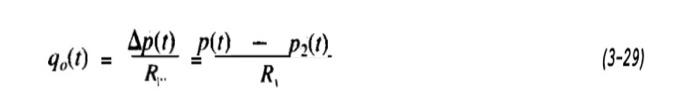
where R_v = resistance to flow at the valve, psi/scfm. We are interested in knowing how the pressure in the tank responds to changes in the inlet flow, q_i(t), and in the valve outlet pressure, p_2(t).
For this process the required relationship is given by a steady state mass balance:
 1 equation, 2 unknowns (qo(t), m(t))
1 equation, 2 unknowns (qo(t), m(t))where:
m(z) = mass of the gas in the tank, Ib
= gas density at standard conditions of 14.7 psia and 60F, lb/(ft^3).
If the pressure in the tank is low, the relationship between the mass of the gas and the pressure is established with the equation of state for perfect gases:
 2 equations, 3 unknowns (p(t))
2 equations, 3 unknowns (p(t))T = absolute temperature in the tank, R
V = volume of tank, ft^3
M = molecular weight of gas
R = perfect gas constant =10.73 (ft3*psia)/(lb mol* R)

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


