Question
Consider the gas-phase equilibrium reaction between water, methane, hydrogen, and carbon dioxide in a variable volume piston that maintains a constant pressure of 10.0 bar
Consider the gas-phase equilibrium reaction between water, methane, hydrogen, and carbon dioxide in a variable volume piston that maintains a constant pressure of 10.0 bar and a constant temperature of 450K. Assume the system starts out-of-equilibrium with 1.0 mol H2O, 0.5 mol CH4, 2.0 mol H2 and 0.5 mol CO2, and that equilibrium is established by the reaction:
2 H2O + CH44 H2 + CO2
Use partition functions and molecular data from NIST to answer the following questions
1. Is the forward reaction between water and methane exothermic or endothermic?
2. What is the standard entropy change for the forward reaction?
3. What is the standard Gibbs Free Energy change for the forward reaction?
4. What is the value of the equilibrium constant, Kp, for this reaction?
5. Based upon the value of the equilibrium constant, will the partial pressure of H2 bel arger or smaller than the initial partial pressure of H2?
6. Will raising the temperature raise or lower the value of Kp?
7. Will raising the external pressure on the piston raise or lower the value of Kp?
IMPORTANT FIRST STEP: You can use the data in the image to answer the questions
USEFUL INFORMATION FOR HYDROGEN GAS:
Rotational constant: B=60.853 cm1
Fundamental vibrational spatial frequency: ~v=4161 cm1
Electronic ground state degeneracy: g=1
Vibrational zero-point energy (in wavenumbers): ~z=2080.6 cm1
Atomization enthalpy at 0 K: D0=432.07 kJ /mol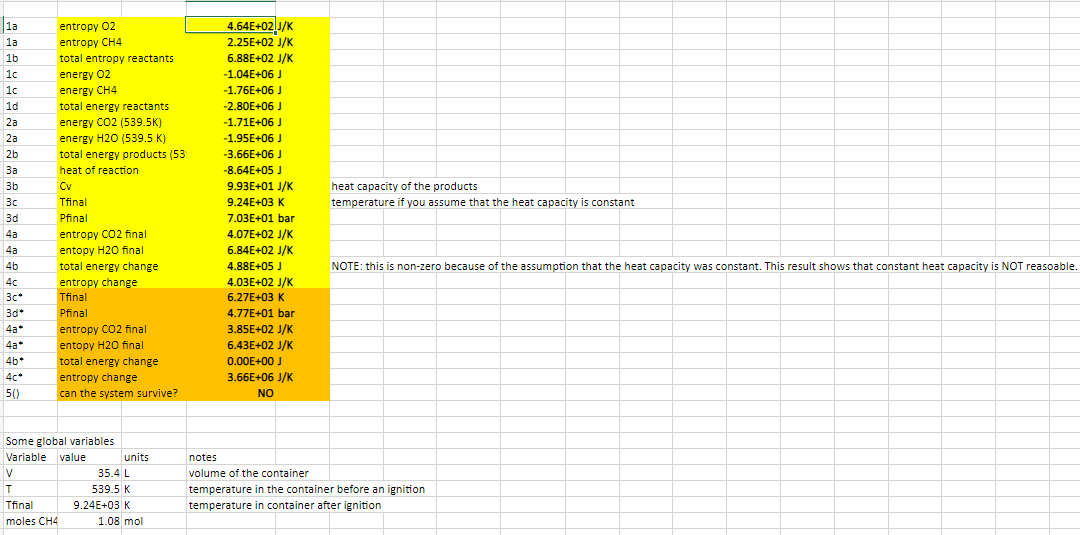
Some global variables Variable value units notes V35.4L volume of the container T 539.5K temperature in the container before an ignition Tfinal 9.24E+03K temperature in container after ignition moles CH41.08mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


