Answered step by step
Verified Expert Solution
Question
1 Approved Answer
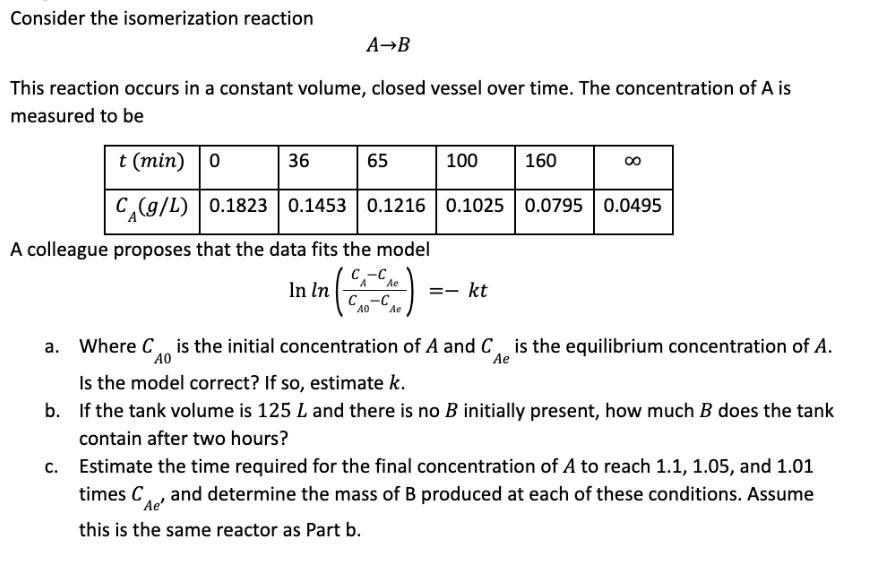
Consider the isomerization reaction A B This reaction occurs in a constant volume, closed vessel over time. The concentration of A is measured to be
Consider the isomerization reaction
This reaction occurs in a constant volume, closed vessel over time. The concentration of is
measured to be
A colleague proposes that the data fits the model
a Where is the initial concentration of A and is the equilibrium concentration of
Is the model correct? If so estimate
b If the tank volume is and there is no initially present, how much does the tank
contain after two hours?
c Estimate the time required for the final concentration of to reach and
times and determine the mass of produced at each of these conditions. Assume
this is the same reactor as Part
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


