Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the NO3-ion which belongs to the Dn point group. The combination of the three RP, orbitals from each oxygen atom (2pz(a)2p()2pz(C)], where the letters
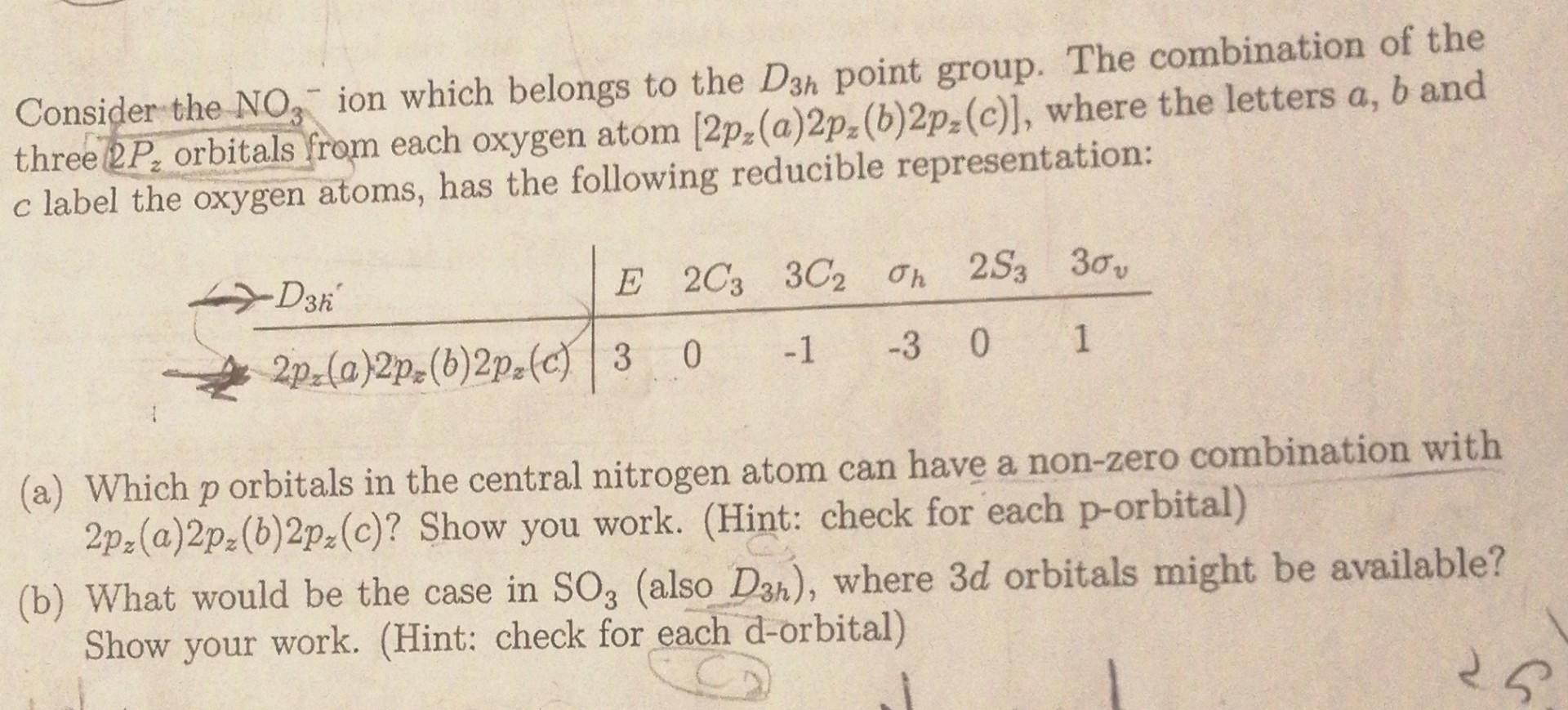
Consider the NO3-ion which belongs to the Dn point group. The combination of the three RP, orbitals from each oxygen atom (2pz(a)2p()2pz(C)], where the letters a, b and c label the oxygen atoms, has the following reducible representation: XD3h E 2C3 3C2 On 25; 30 -1 0 -30 1 2pz(a)2pz(b)2pz(0) 3 (a) Which p orbitals in the central nitrogen atom can have a non-zero combination with 2pz(a)2pz(b)2pz(c)? Show you work. (Hint: check for each p-orbital) (b) What would be the case in SO2 (also D3n), where 3d orbitals might be available? Show your work. (Hint: check for each d-orbital)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


