Answered step by step
Verified Expert Solution
Question
1 Approved Answer
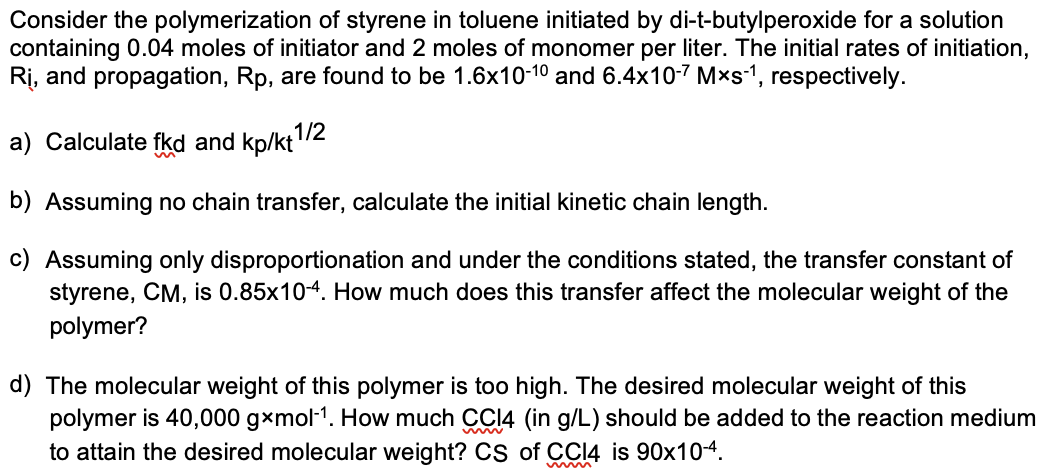
Consider the polymerization of styrene in toluene initiated by di - t - butylperoxide for a solution containing 0 . 0 4 moles of initiator
Consider the polymerization of styrene in toluene initiated by ditbutylperoxide for a solution
containing moles of initiator and moles of monomer per liter. The initial rates of initiation,
and propagation, are found to be and respectively.
a Calculate fkd and kpkt
b Assuming no chain transfer, calculate the initial kinetic chain length.
c Assuming only disproportionation and under the conditions stated, the transfer constant of
styrene, is How much does this transfer affect the molecular weight of the
polymer?
d The molecular weight of this polymer is too high. The desired molecular weight of this
polymer is How much in gL should be added to the reaction medium
to attain the desired molecular weight? of is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


