Answered step by step
Verified Expert Solution
Question
1 Approved Answer
consider the reaction 4NH3 + 502 4NO + 6H20, , and in this reaction 2.55 g of NH3 reacted with 8.50 g of 0. calculate
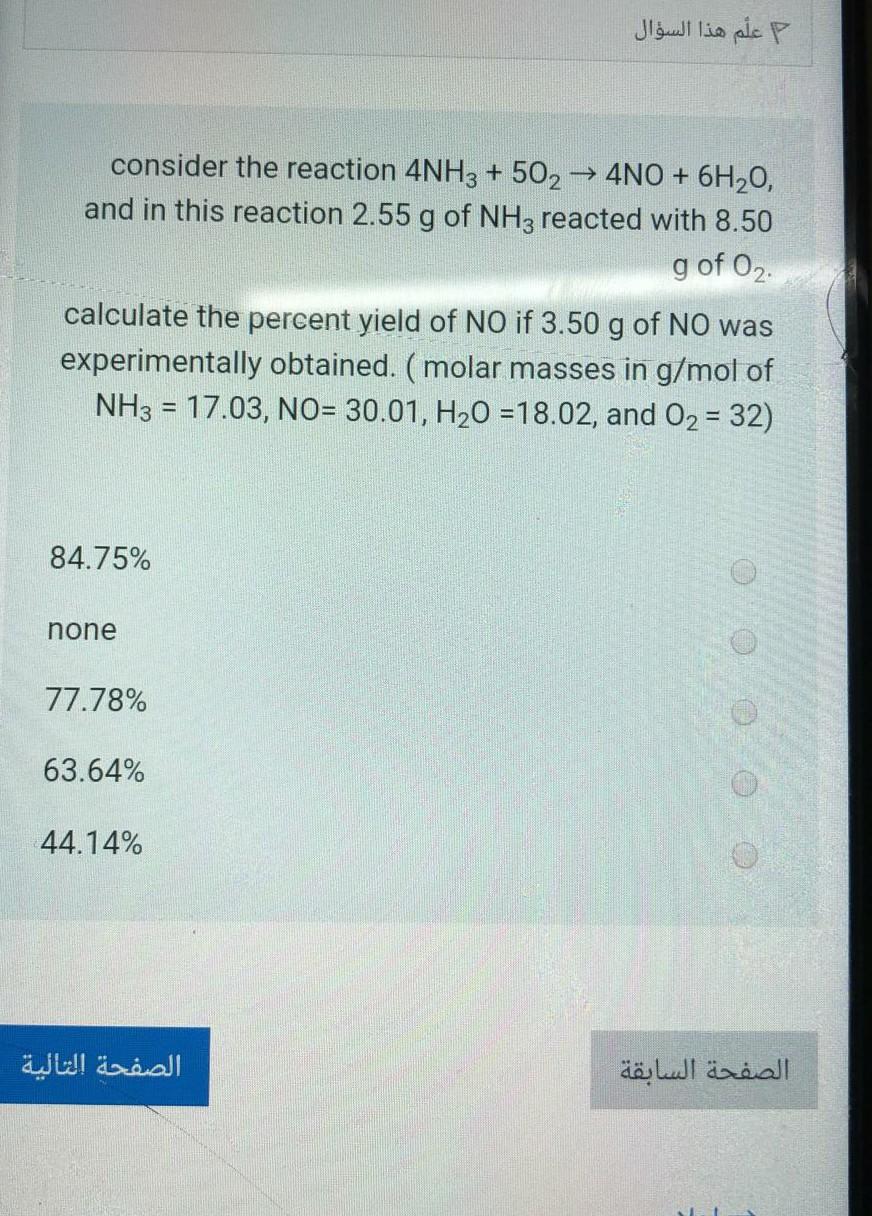
consider the reaction 4NH3 + 502 4NO + 6H20, , and in this reaction 2.55 g of NH3 reacted with 8.50 g of 0. calculate the percent yield of NO if 3.50 g of NO was experimentally obtained. (molar masses in g/mol of NH3 = 17.03, NO= 30.01, H20 =18.02, and 02 = 32) 84.75% none 77.78% 63.64% 44.14%
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


