Question
Consider the reaction CO(g) + 3H(g)CH(g) + HO(g) for which AH-206.1 k) and AS-214.7 J/K at 298.15 K. (1) Calculate the entropy change of
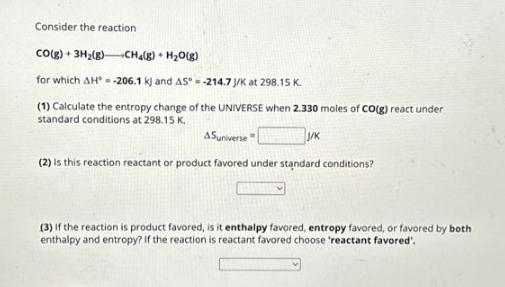
Consider the reaction CO(g) + 3H(g)CH(g) + HO(g) for which AH-206.1 k) and AS-214.7 J/K at 298.15 K. (1) Calculate the entropy change of the UNIVERSE when 2.330 moles of CO(g) react under standard conditions at 298.15 K. J/K Suniverse (2) Is this reaction reactant or product favored under standard conditions? (3) if the reaction is product favored, is it enthalpy favored, entropy favored, or favored by both enthalpy and entropy? If the reaction is reactant favored choose 'reactant favored'.
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



