Question
Consider the reaction: Determine the product temperature if the reaction is adiabatic process, complete, and the pressure is at 1 bar. Consider the following conditions
Consider the reaction:
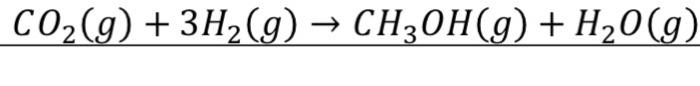
Determine the product temperature if the reaction is adiabatic process, complete, and the pressure is at 1 bar.
Consider the following conditions :
a) Both reactants are in reduced temperature > 25C and in stoichiometric quantities.
b) Both reactants are in reduced temperature at 25C and in stoichiometric quantities.
c) Both of the reactants are in reduced temperature at 25C; one of the reactants is in excess. Note: create two calculations to determine which is the excess.
c.1) which of the reactants is in excess?
Please help me, i will give upvote if answers are all correct. Thank you!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


