Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the reaction: HCl(ag) + NH, (ag) = NH,CI(aq) Where 20.00 mL of 0.100 M NH, (aq) is added to 10.00 mL of 0.200
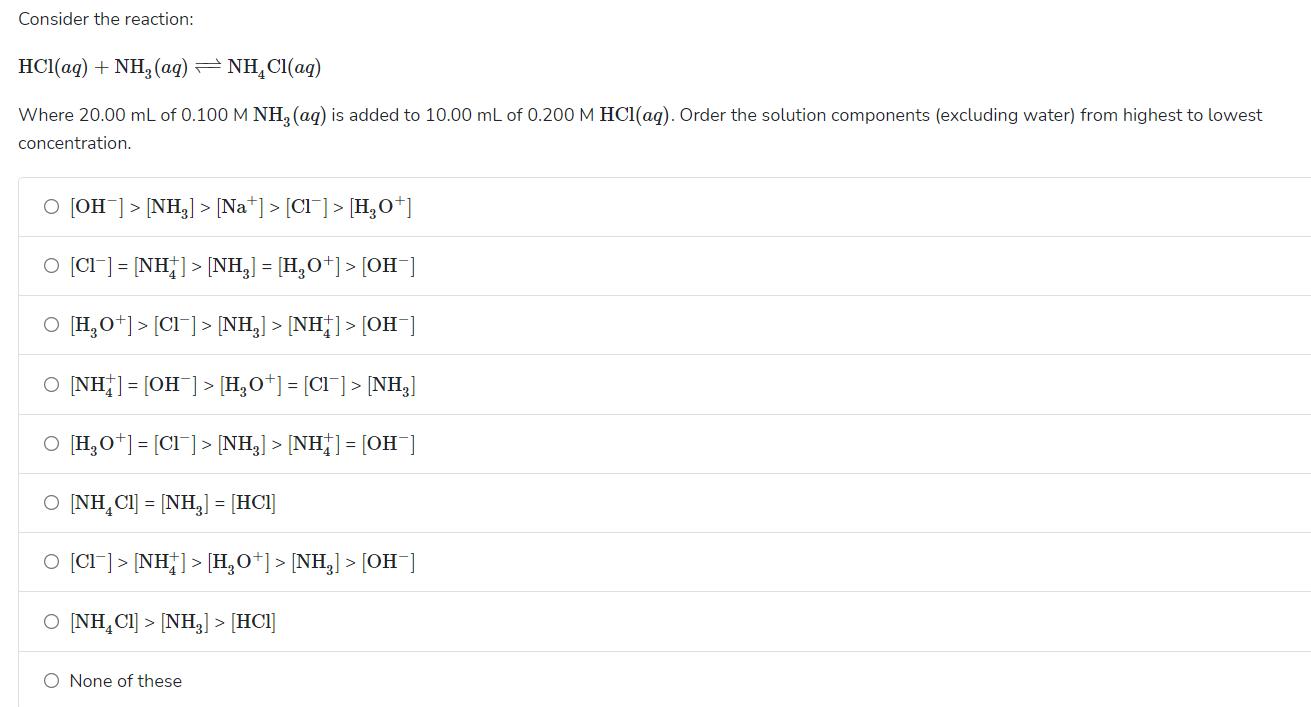
Consider the reaction: HCl(ag) + NH, (ag) = NH,CI(aq) Where 20.00 mL of 0.100 M NH, (aq) is added to 10.00 mL of 0.200 M HCl(aq). Order the solution components (excluding water) from highest to lowest concentration. O [OH ]> [NH,] > [Na*] > [Cl]> [H,0*] O (CI] = [NH] > [NH,] = [H,O*]> [OH] O [H,0*] > [Cl]> [NH,] > [NH]> [OH ] O INH]= [OH ]> [H,O*]= [CI] > [NH,] O [H,0*] = [CI]> [NH,] > [NH] = [OH ] O INH,CI] = [NH,] = [HCI] O [CI]> [NH]> [H,O*] > [NH,] > [OH ] O INH,CI] > [NH,] > [HCl] O None of these
Step by Step Solution
★★★★★
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635f98888cf1d_232522.pdf
180 KBs PDF File
635f98888cf1d_232522.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


