Answered step by step
Verified Expert Solution
Question
1 Approved Answer
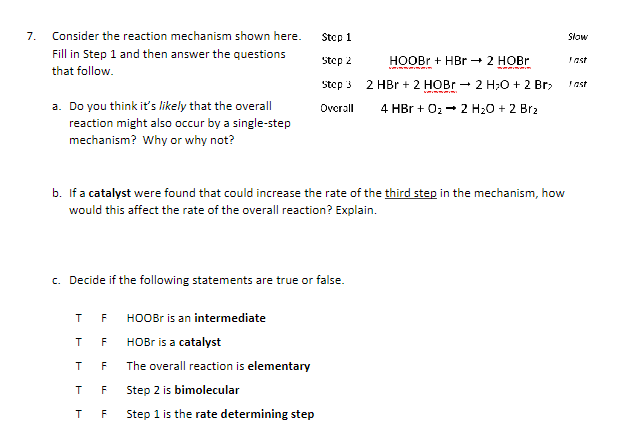
Consider the reaction mechanism shown here. Fill in Step 1 and then answer the questions that follow. a . Do you think it's likely that
Consider the reaction mechanism shown here.
Fill in Step and then answer the questions
that follow.
a Do you think it's likely that the overall
reaction might also occur by a singlestep
mechanism? Why or why not?
Stcp
HOBr
last
step HOBr
last
Overall
b If a catalyst were found that could increase the rate of the third step in the mechanism, how
would this affect the rate of the overall reaction? Explain.
c Decide if the following statements are true or false.
T HOOBr is an intermediate
T HOBr is a catalyst
The overall reaction is elementary
T Step is bimolecular
T F Step is the rate determining step
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


