
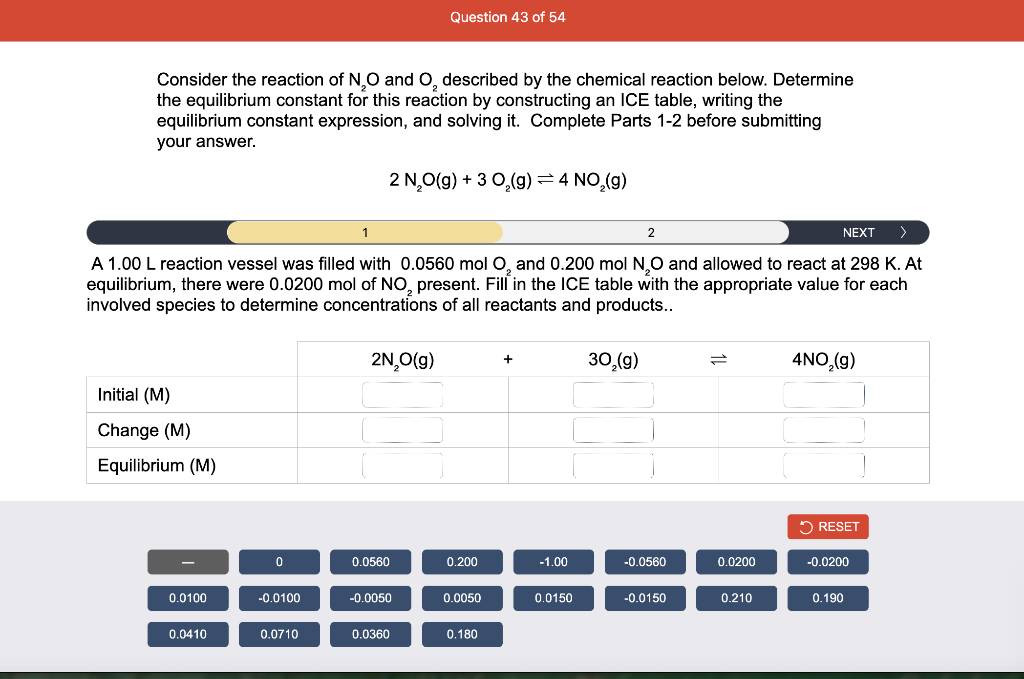
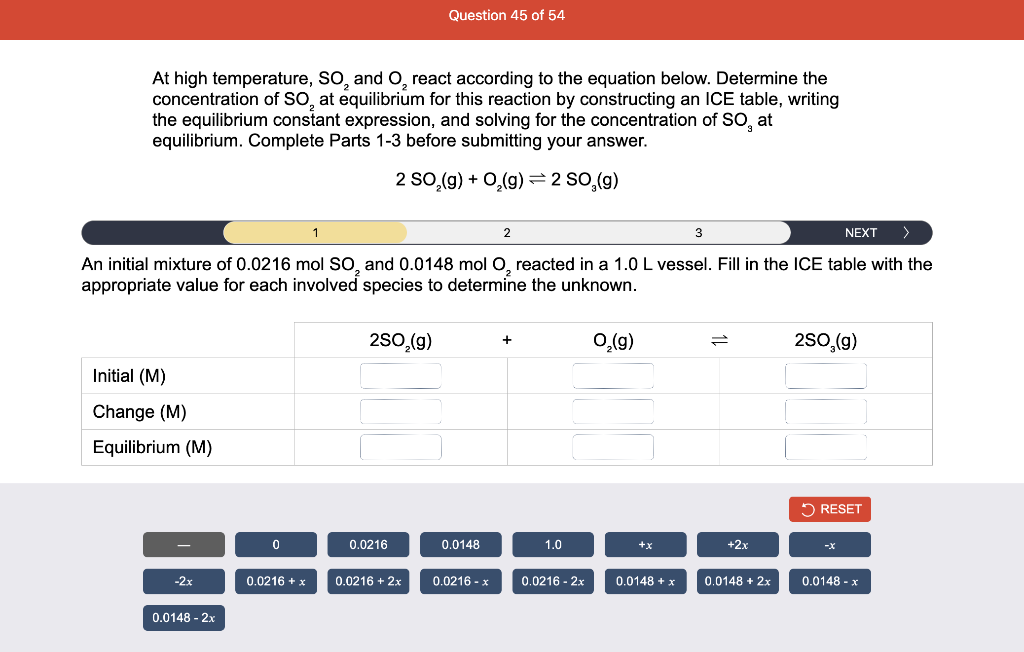
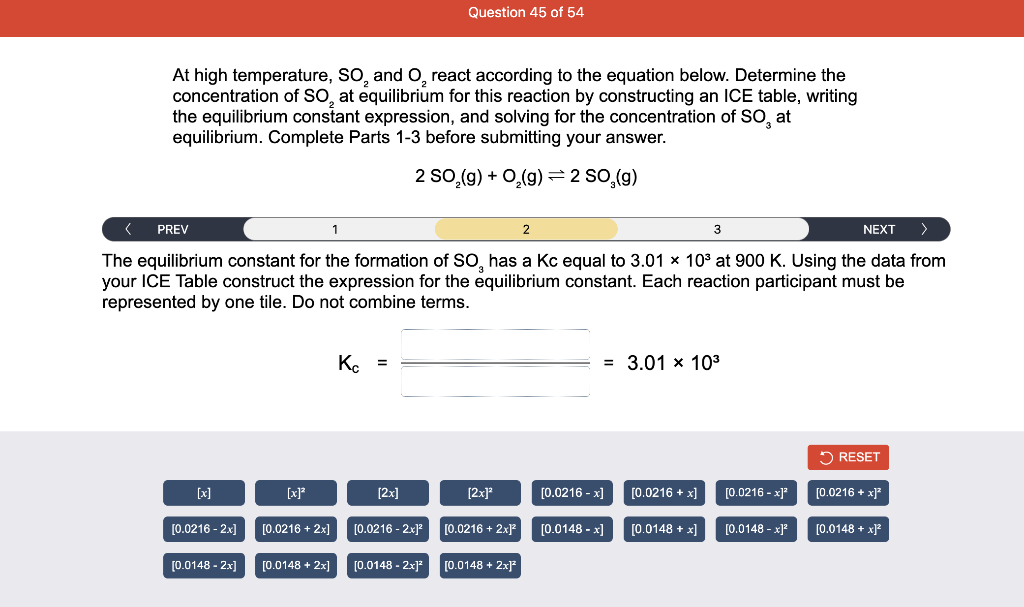


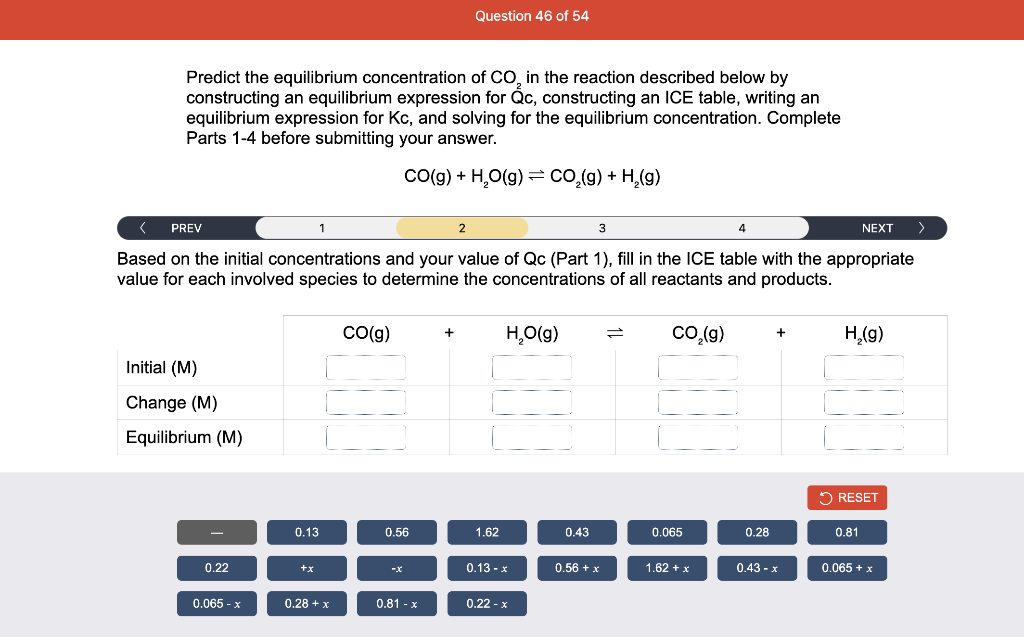

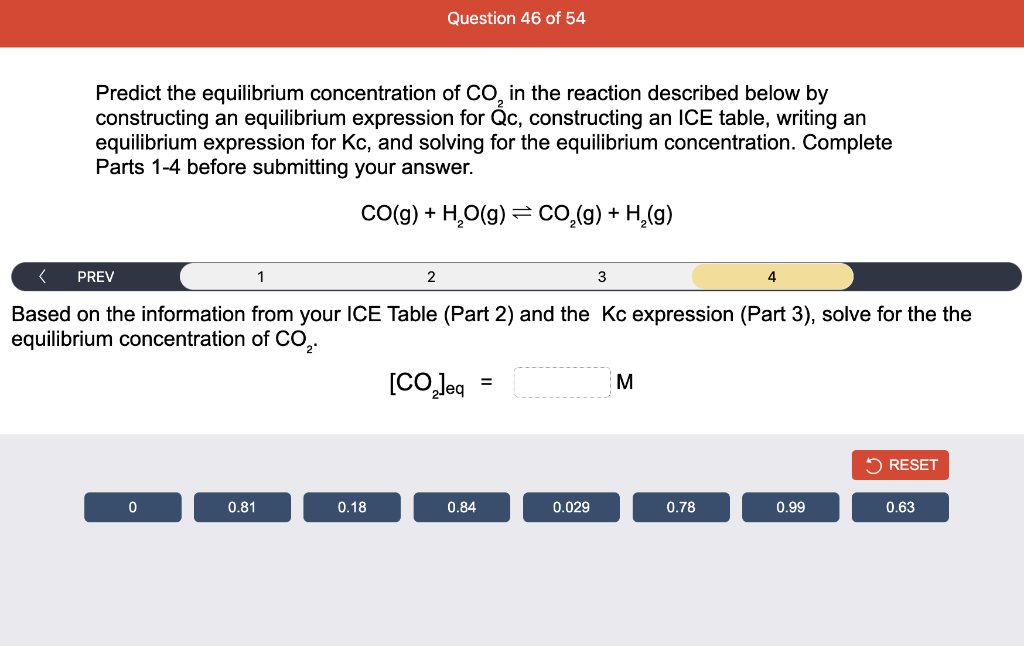

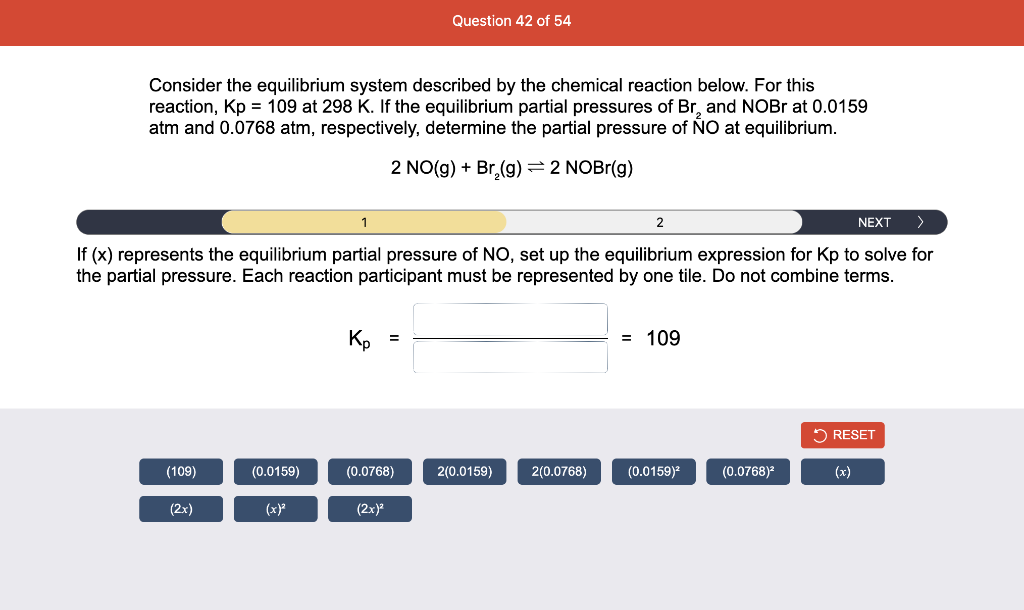
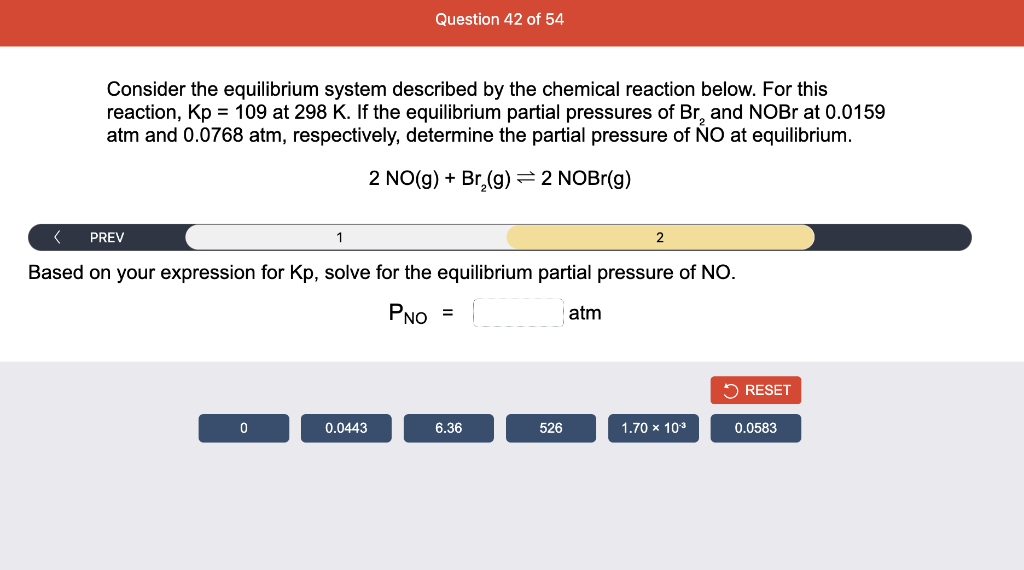
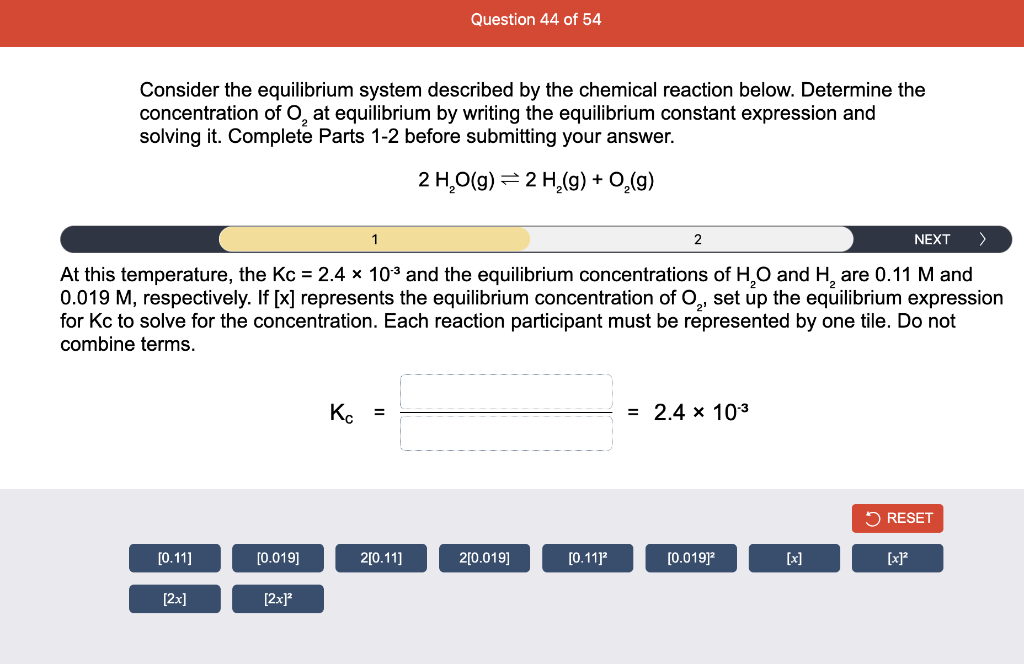
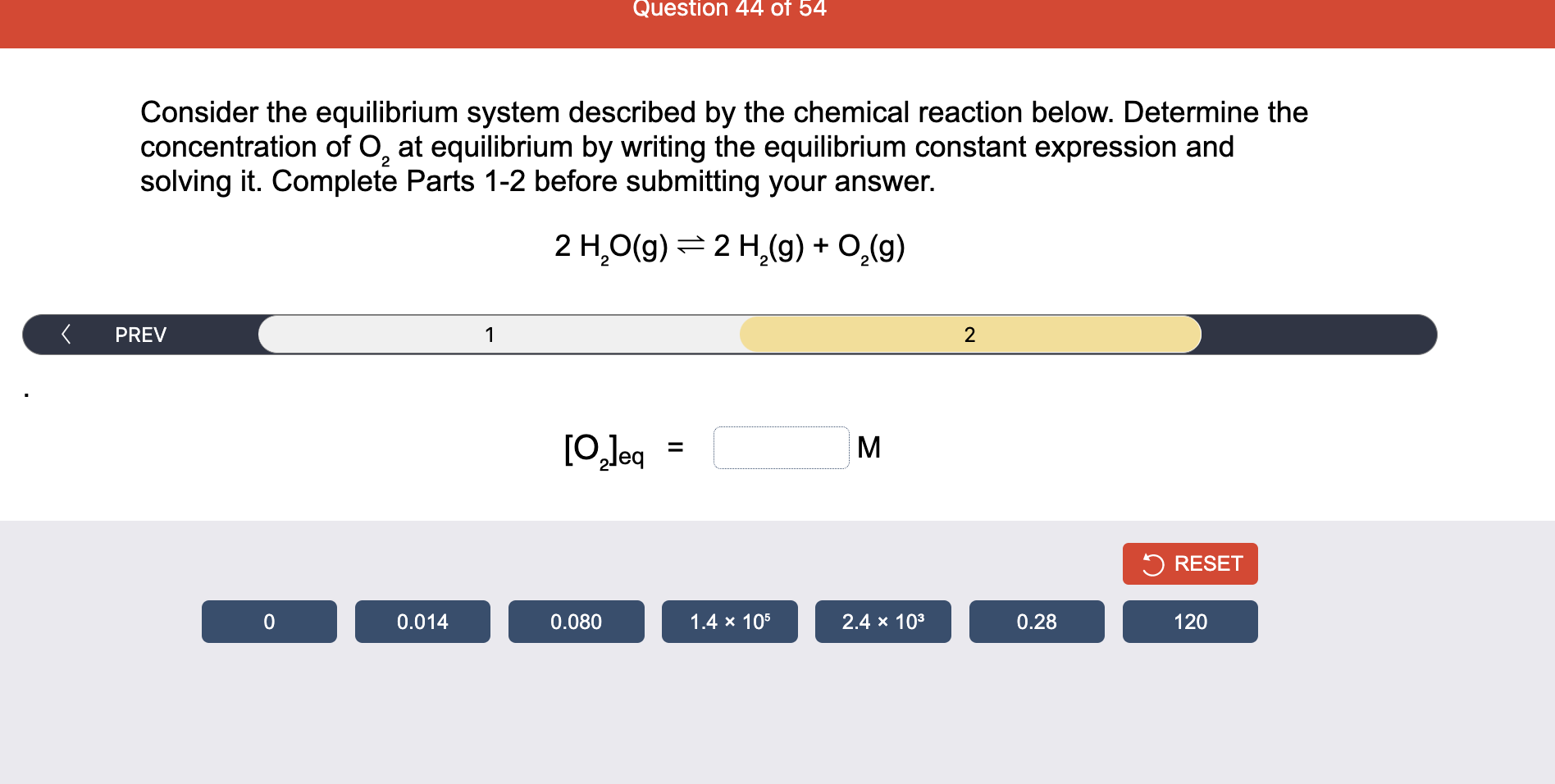
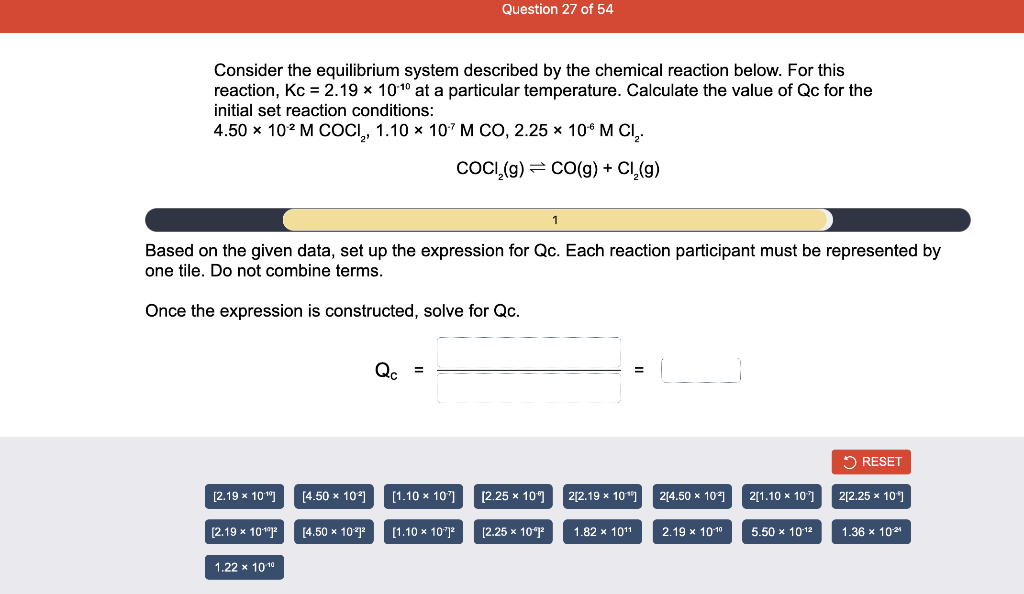
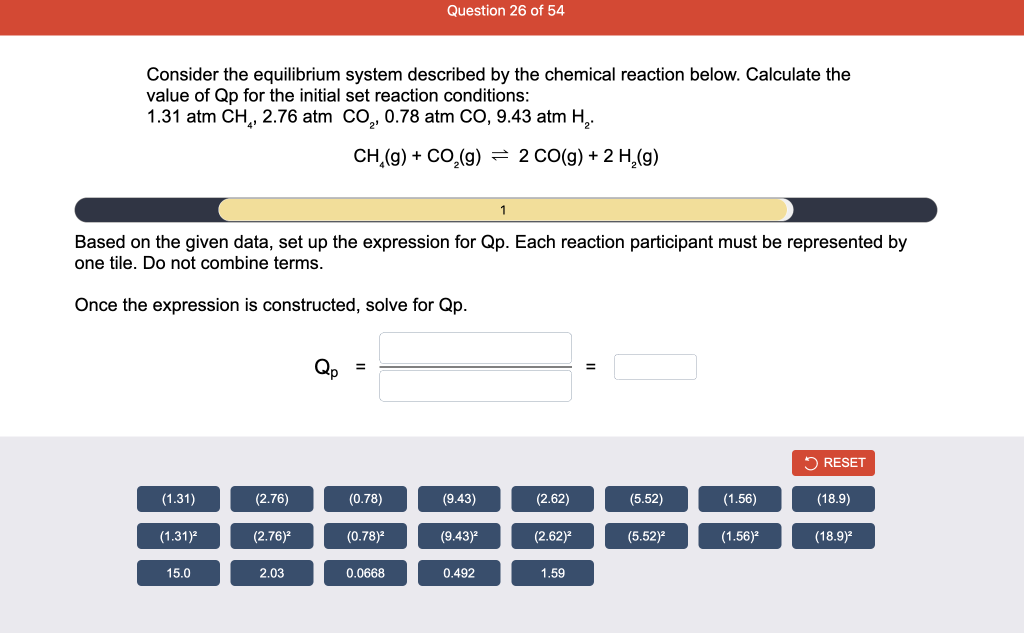
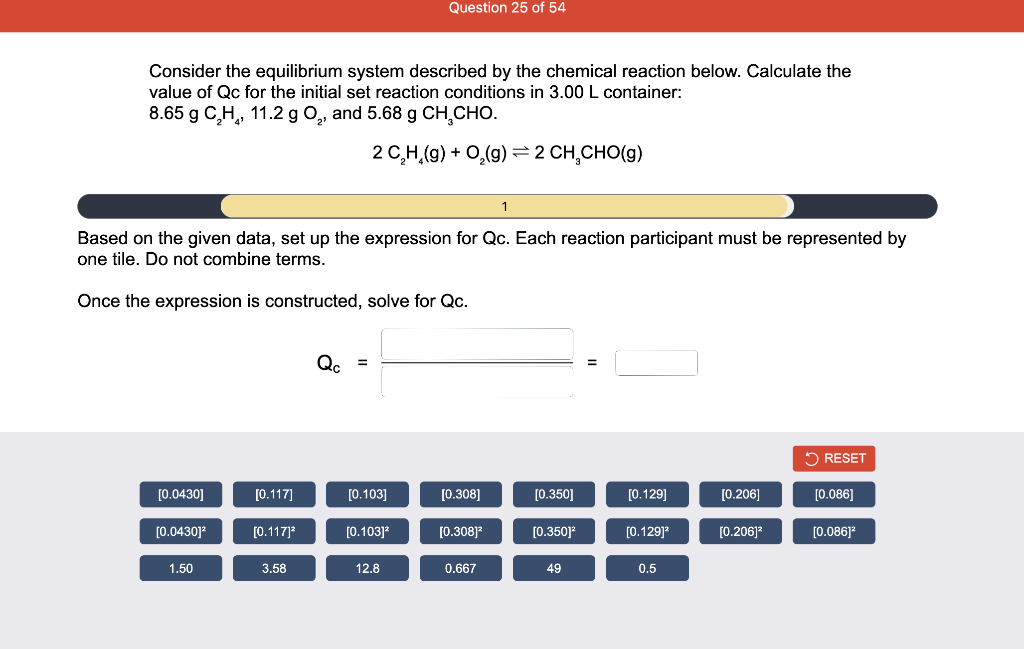
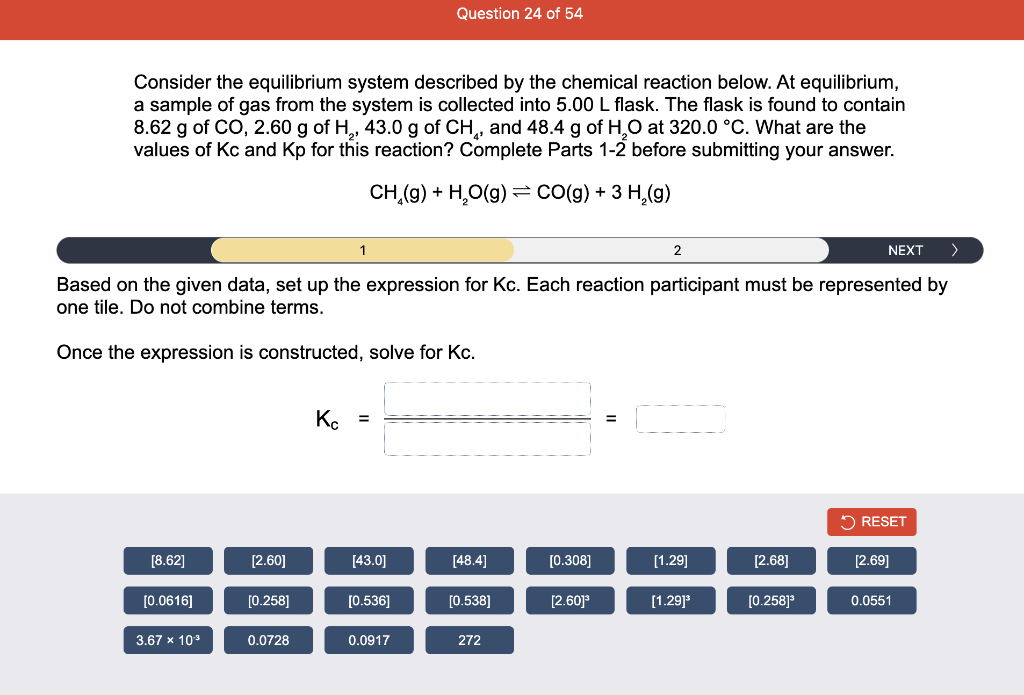
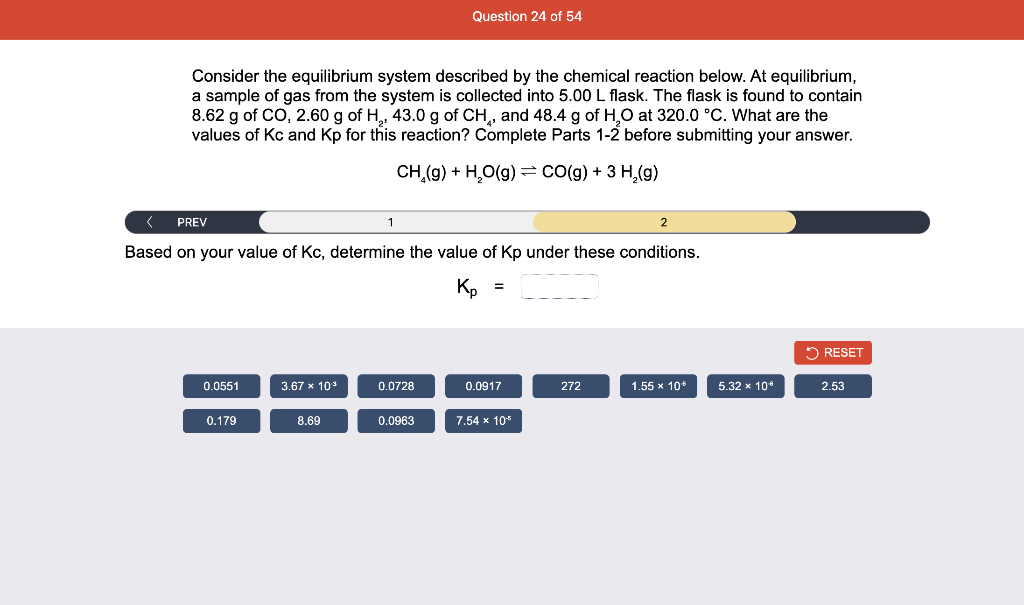
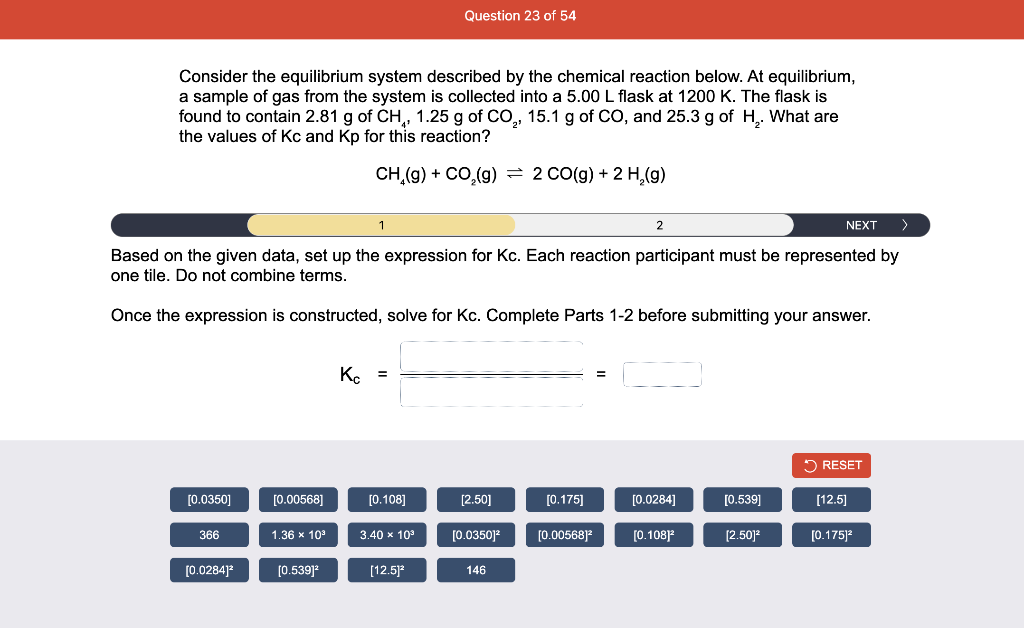
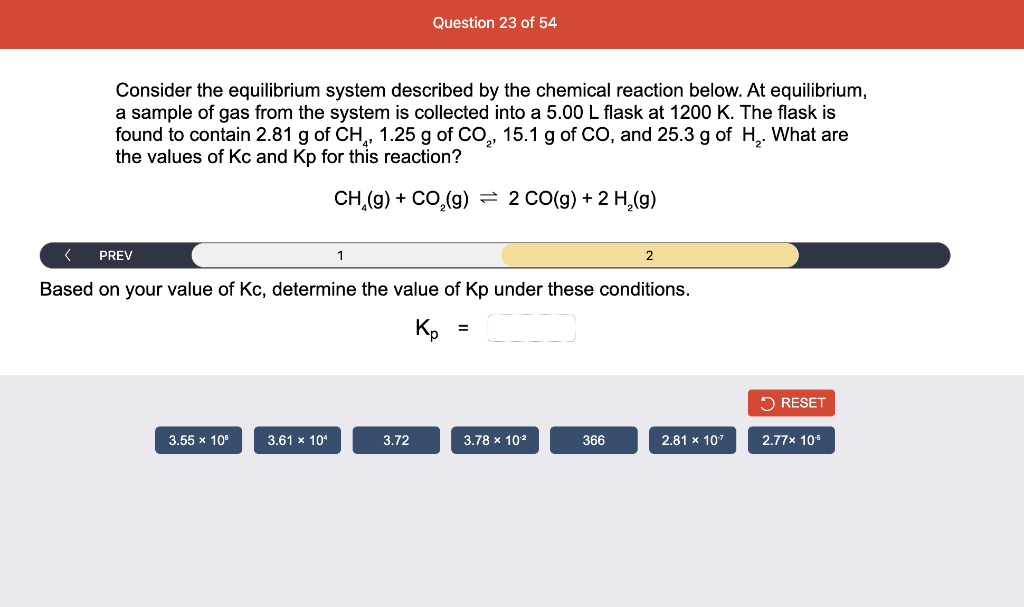
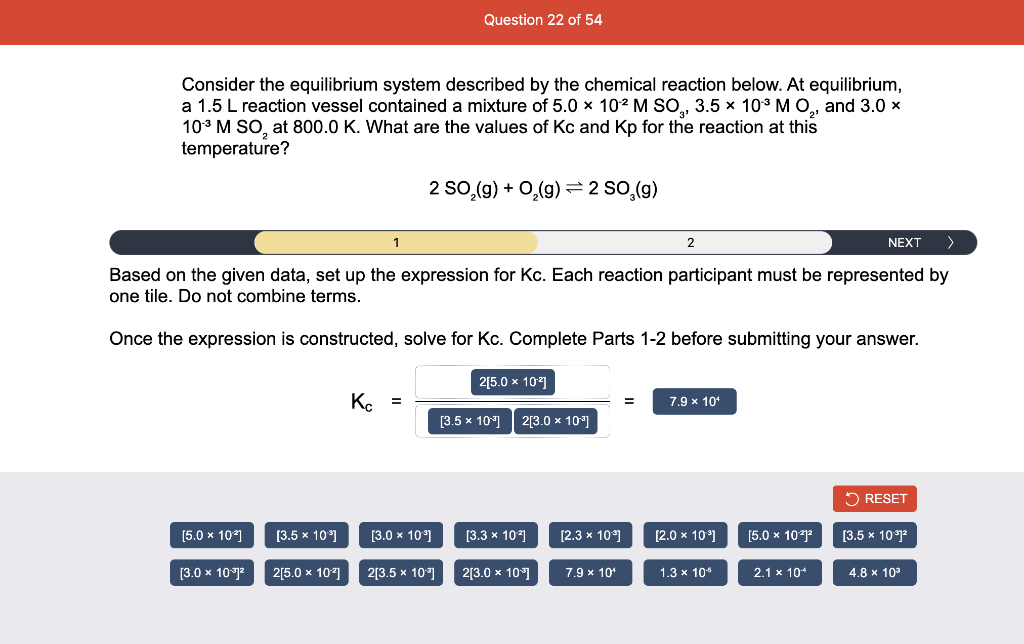
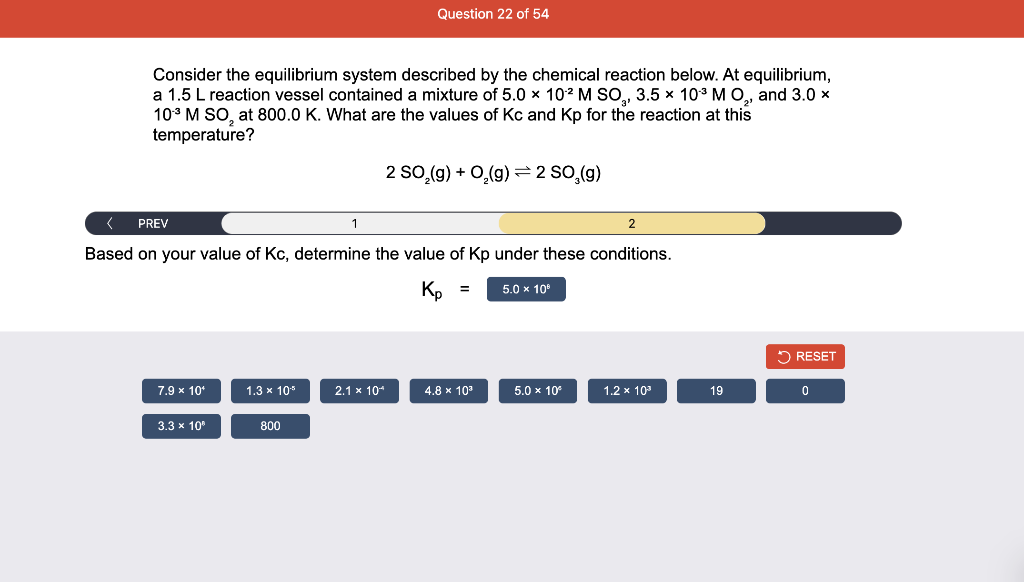
Consider the reaction of N2O and O2 described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. 2N2O(g)+3O2(g)4NO2(g) Kc= Consider the reaction of N2O and O2 described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. 2N2O(g)+3O2(g)4NO2(g) A 1.00L reaction vessel was filled with 0.0560molO2 and 0.200molN2O and allowed to react at 298K. At equilibrium, there were 0.0200mol of NO2 present. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products.. At high temperature, SO2 and O2 react according to the equation below. Determine the concentration of SO2 at equilibrium for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving for the concentration of SO3 at equilibrium. Complete Parts 1-3 before submitting your answer. 2SO2(g)+O2(g)2SO3(g) An initial mixture of 0.0216molSO2 and 0.0148molO2 reacted in a 1.0L vessel. Fill in the ICE table with the appropriate value for each involved species to determine the unknown. At high temperature, SO2 and O2 react according to the equation below. Determine the concentration of SO2 at equilibrium for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving for the concentration of SO3 at equilibrium. Complete Parts 1-3 before submitting your answer. 2SO2(g)+O2(g)2SO3(g) PREV \begin{tabular}{c|c} 3 & NEXT > \end{tabular} The equilibrium constant for the formation of SO3 has a Kc equal to 3.01103 at 900K. Using the data from our ICE Table construct the expression for the equilibrium constant. Each reaction participant must be epresented by one tile. Do not combine terms. Kc==3.01103 At high temperature, SO2 and O2 react according to the equation below. Determine the concentration of SO2 at equilibrium for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving for the concentration of SO3 at equilibrium. Complete Parts 1-3 before submitting your answer. 2SO2(g)+O2(g)2SO3(g) ased on the initial concentrations and your value of Qc (Part 1), fill in the ICE table with the appropriate lue for each involved species to determine the concentrations of all reactants and products. Predict the equilibrium concentration of CO2 in the reaction described below by constructing an equilibrium expression for Qc, constructing an ICE table, writing an equilibrium expression for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before submitting your answer. CO(g)+H2O(g)CO2(g)+H2(g) > \end{tabular} The value of Kc is 5.1 at a temperature of 700K. Based on your ICE table (Part 2), set up the equilibrium expression for Kc in order to determine concentrations of all species. Each reaction participant must be represented by one tile. Do not combine terms. Predict the equilibrium concentration of CO2 in the reaction described below by constructing an equilibrium expression for Qc, constructing an ICE table, writing an equilibrium expression for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before submitting your answer. CO(g)+H2O(g)CO2(g)+H2(g) sed on the information from your ICE Table (Part 2) and the Kc expression (Part 3), solve for the the uilibrium concentration of CO2. [CO2]eq=M For the reaction: 2A(g)+B(s)2C(s)+D(g)Kp=8210 At 298K in a 10.0L vessel, the known equilibrium values are as follows: 0.031atm of A,0.22mol of B, and 10.5mol of C. What is the equilibrium partial pressure of D ? Consider the equilibrium system described by the chemical reaction below. For this reaction, Kp=109 at 298K. If the equilibrium partial pressures of Br2 and NOBr at 0.0159 atm and 0.0768atm, respectively, determine the partial pressure of NO at equilibrium. 2NO(g)+Br2(g)2NOBr(g) 2NEXT> If (x) represents the equilibrium partial pressure of NO, set up the equilibrium expression for Kp to solve for the partial pressure. Each reaction participant must be represented by one tile. Do not combine terms. Consider the equilibrium system described by the chemical reaction below. For this reaction, Kp=109 at 298K. If the equilibrium partial pressures of Br2 and NOBr at 0.0159 atm and 0.0768atm, respectively, determine the partial pressure of NO at equilibrium. 2NO(g)+Br2(g)2NOBr(g) Consider the equilibrium system described by the chemical reaction below. Determine the concentration of O2 at equilibrium by writing the equilibrium constant expression and solving it. Complete Parts 1-2 before submitting your answer. 2H2O(g)2H2(g)+O2(g) t this temperature, the Kc=2.4103 and the equilibrium concentrations of H2O and H2 are 0.11M and .019M, respectively. If [x] represents the equilibrium concentration of O2, set up the equilibrium expressio or Kc to solve for the concentration. Each reaction participant must be represented by one tile. Do not ombine terms. Kc=:=2.4103 Consider the equilibrium system described by the chemical reaction below. Determine the concentration of O2 at equilibrium by writing the equilibrium constant expression and solving it. Complete Parts 1-2 before submitting your answer. 2H2O(g)2H2(g)+O2(g) PREV 1 2 [O2]eq=M Consider the equilibrium system described by the chemical reaction below. For this reaction, Kc=2.191010 at a particular temperature. Calculate the value of Qc for the initial set reaction conditions: 4.50102MCOCl2,1.10107MCO,2.25106MCl2.COCl2(g)CO(g)+Cl2(g) Based on the given data, set up the expression for Qc. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Qc. Qc== Consider the equilibrium system described by the chemical reaction below. Calculate the value of Qp for the initial set reaction conditions: 1.31atmCH4,2.76atmCO2,0.78atmCO,9.43atmH2. CH4(g)+CO2(g)2CO(g)+2H2(g) Based on the given data, set up the expression for Qp. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Qp. Qp=1= Consider the equilibrium system described by the chemical reaction below. Calculate the value of Qc for the initial set reaction conditions in 3.00L container: 8.65gC2H4,11.2gO2, and 5.68gCH3CHO. 2C2H4(g)+O2(g)2CH3CHO(g) Based on the given data, set up the expression for Qc. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Qc. Qc= Consider the equilibrium system described by the chemical reaction below. At equilibrium, a sample of gas from the system is collected into 5.00L flask. The flask is found to contain 8.62g of CO,2.60g of H2,43.0g of CH4, and 48.4g of H2O at 320.0C. What are the values of Kc and Kp for this reaction? Complete Parts 12 before submitting your answer. CH4(g)+H2O(g)CO(g)+3H2(g) 3ased on the given data, set up the expression for Kc. Each reaction participant must be represented by ne tile. Do not combine terms. Ince the expression is constructed, solve for Kc. Kc=: Consider the equilibrium system described by the chemical reaction below. At equilibrium, a sample of gas from the system is collected into 5.00L flask. The flask is found to contain 8.62g of CO,2.60g of H2,43.0g of CH4, and 48.4g of H2O at 320.0C. What are the values of Kc and Kp for this reaction? Complete Parts 1-2 before submitting your answer. CH4(g)+H2O(g)CO(g)+3H2(g)





























