Question
Consider the reaction sequence from P to Q shown below. The overall yield of the major product Q from P is 75%. What is
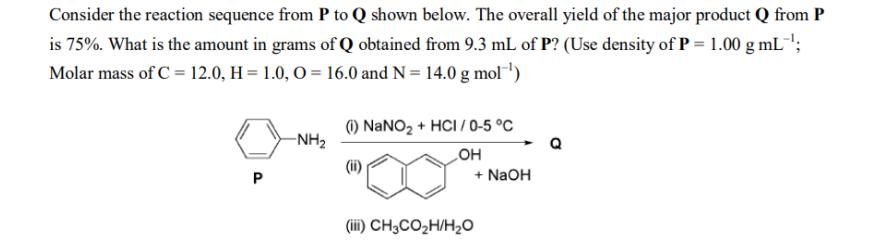
Consider the reaction sequence from P to Q shown below. The overall yield of the major product Q from P is 75%. What is the amount in grams of Q obtained from 9.3 mL of P? (Use density of P = 1.00 g mL-; Molar mass of C = 12.0, H = 1.0, O=16.0 and N = 14.0 g mol-) P --NH (1) NaNO + HCI/0-5 C OH + NaOH (iii) CH3COH/HO Q
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Marc Loudon
5th edition
981519431, 978-0981519449, 098151944X, 978-0-98151943, 978-0981519432
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



