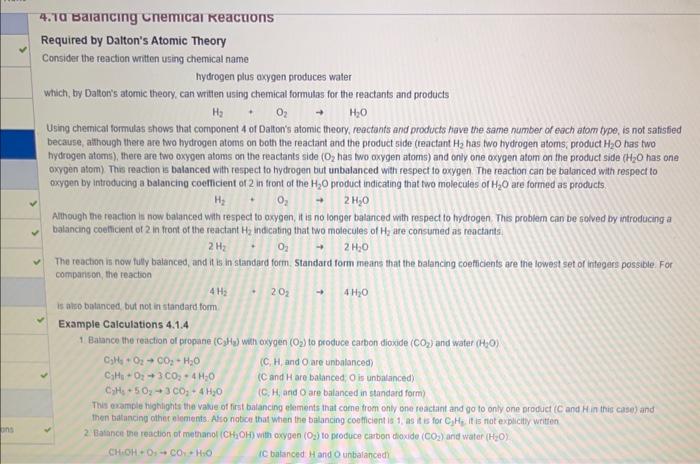
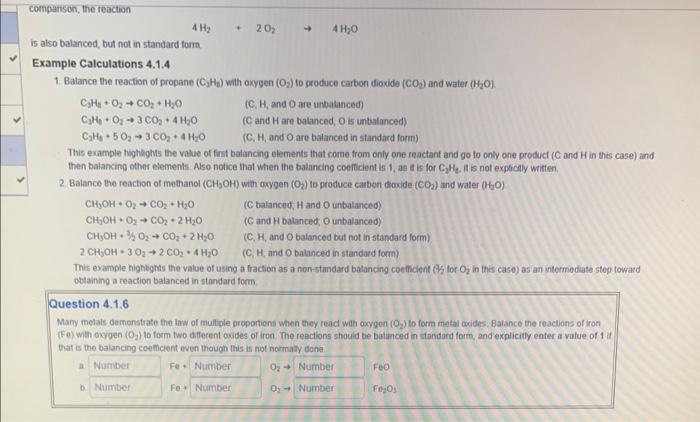
Consider the reaction written using chemical name hydrogen plus axygen produces water which, by Datton's atomic theory, can written using chemical formutas for the reactants and products Using cherrical formulas shows that component 4 of Dalton's atomic theory, reactants and products have the same number of each atom type, is not satisfied because, athough there are two hydrogen atoms on both the reaclant and the product side (reactant H2 has fwo hydrogen atoms, product H2O has fwo hydrogen atoms), there are two oxygen atoms on the reactants side ( O2 has two oxygen atoms) and onty one oxygen atom on the product side ( H2O has one oxven atom) This reaction is balanced with respect to hydrogen but unbalanced with respect to oxygen. The reaction can be batonced with respect to oxygen by introducing a balancing coefficient of 2in front of the H2O product indicating that two molecules of H2O are formed as products. H2+O22H2O Alithough the reaction is now balanced with respect to axygen, it is no longer balanced with respect to tydrogen. This problem can be folved by introducing a balancing coefficient of 2 in front of the reactant H2 indicating that two molecules of H2 are consumed as reactants. 2H2O32H2O The reaction is now fully balanced, and it is in standard form. Standard form means that the balancing coefticients are the lowest set of integers possible. For comparison, the reaction is aho bulanced, but not in standard form. Example Calculations 4.1.4 1. Batance the readion of propane (C2H2) with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). C2H4+O2CO2+H2OC2H1+O2+3CO2+4H2OC2H3+5O2+3CO2+4H2O (C, H,andO are unbalanced) (C and H are balinced, O is unbalanced) (C. H, and O are balanced in standard form) This oxample highights the value of first balancing ehements that corne trom only one reactant and go to onty one prodict ( C and H in this case) and then batancing oiher eiements. Atio natice that when the balancing cootticient is 1, as it is for C3H2 it is not explictly written 2 Balance the reaction of methanol (CH2OH) with oxygen (O2) to poduce carbon dioxide (CO2) and water (H2O). CH+OH+O3CO2H2O IC batanced H and O unbalanced) C3H1+O2+CO2+H2OC3H4+O2+3CO2+4H2OC2H4+5O23CO2+4H2O (C, H, and O are unbatanced) (C and H are balanced, O is untialanced) This example highaghts the vatue of frst balaincing elements that corne from only one reactant and go to only one product ( C and H in this case) and 2. Balance the reaction of methanol (CH2OH) with oxygen (O2) to produce cambon dooside (CO2) and water (H2O) CH2OH+O2CO2+H2OCH2OH+O2CO2+2H2OCHH3OH+3S2O2CO2+2H2O2CH2OH+3O22CO2+4H2O (C talanced, H and O unbalanced) ( C and H batanced, O unbalanced) (C, H, and O balanced but not in standard form) This example highbights the value of using a fraction as a non-standard batancing coeffient (H2 tor O2 in this case) as an intermediate step toward obtaining a reaction balanced in standard form. Question 4.1.6 Mary melats demanstate the inw of multiple broperiont when they react with oygon (O2) to form metal weides. Balance the reactions of iron (F e) with oxyoen (O2) to form two different cuides of iron The reactions should be batanced in standard form, and explicitly enter a vatue of 1 it that is the balancing coetficient even though this is not normaly vone