Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the reduction of hydroxyl radical, OH, according to the reaction H3O+ + OH + e- 2 H2O for which E0 = +2.72 V at
Consider the reduction of hydroxyl radical, OH, according to the reaction H3O+ + OH + e- 2 H2O for which E0 = +2.72 V at pH 0. (a) What E0 value is expected at neutral pH 7? (b) Now compare this to the much less favorable direct reduction of hydronium, H3O+. H3O+ + e- H + H2O for which E0 = -2.10 V at pH 0. By comparing these reactions, estimate the Gibbs free energy of bond formation for HO-H, Delta 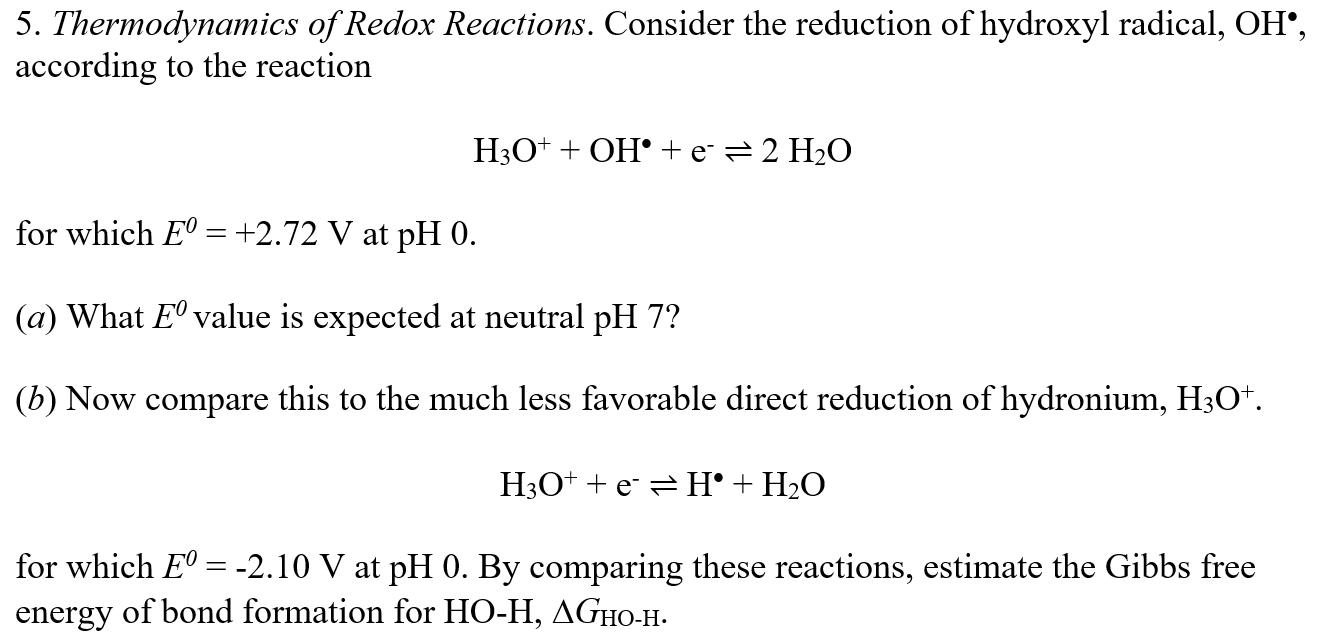 GHO-H.
GHO-H.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


