Question
Consider the strong electrolytes Zm, Xn,Um, Yp and Vm Xn. Limiting molar conductivity (A) m of UY and VX are 250 and 440 S
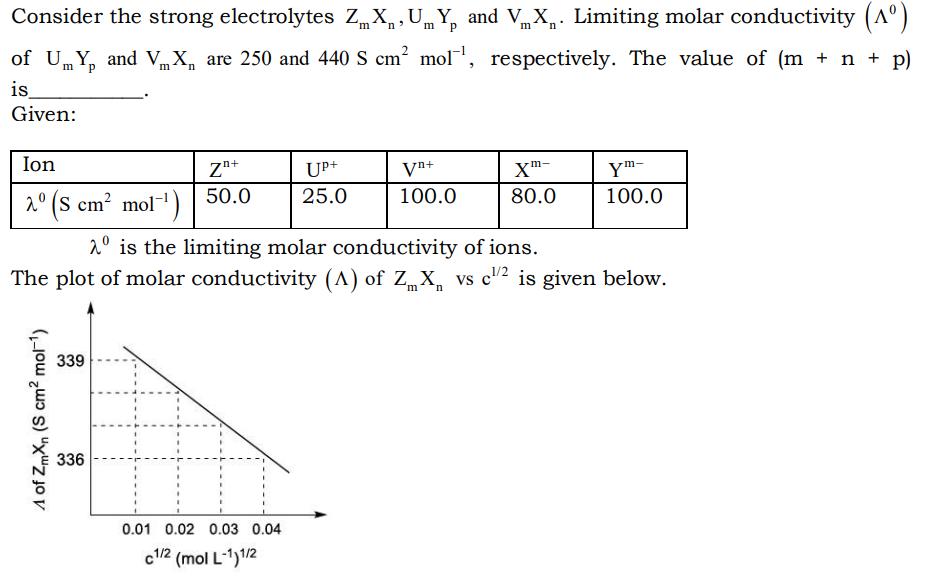
Consider the strong electrolytes Zm, Xn,Um, Yp and Vm Xn. Limiting molar conductivity (A) m of UY and VX are 250 and 440 S cm mol, respectively. The value of (m + n + p) p m is_ Given: Ion 2 (S cm mol-) A of ZmXn (S cm mol-) 339 Zn+ 50.0 336 is the limiting molar conductivity of ions. The plot of molar conductivity (A) of ZX vs c/2 is given below. n H UP+ 25.0 0.01 0.02 0.03 0.04 c1/2 (mol L-1)1/2 Vn+ 100.0 Xm- 80.0 Ym- 100.0
Step by Step Solution
3.54 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Understanding Basic Statistics
Authors: Charles Henry Brase, Corrinne Pellillo Brase
6th Edition
978-1133525097, 1133525091, 1111827028, 978-1133110316, 1133110312, 978-1111827021
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



